How to Cite | Publication History | PlumX Article Matrix
Review of Covid-19's Current Development in Therapeutic and Diagnostic Techniques
Keerthika S1* , Kowsalya M1
, Kowsalya M1 , R Kameswaran2
, R Kameswaran2 and N Venkateswaramurthy1
and N Venkateswaramurthy1
1Department of Pharmacy Practice, JKKN College of Pharmacy, Kumarapalayam, Tamil Nadu, India.
2Department of Pharmacy Practice, Pushpagiri college of Pharmacy, Thiruvalla, Kerala, India.
Corresponding Author E-mail: keerthikask125@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3195
ABSTRACT: The severe acute respiratory syndrome coronavirus (SARS-CoV-2), a novel coronavirus that is related to SARS-CoV-2 and the Middle East respiratory disease coronavirus, has spread widely, prompting the World Health Organisation to declare a pandemic. The disease caused by the SARS-CoV-2, known as COVID-19, has flu-like symptoms that can become serious and expose people to higher risk. At least 64,897,870 COVID-19 cases and 1,500,271 fatalities associated with it were reported globally as of December 3, 2020. SARS-CoV-2 is one of three highly virulent coronaviruses that pose a global threat to public health. The purpose of this study is to the most recent methods for diagnosing and treating COVID-19. Real-time reverse transcription-PCR (RT-PCR) is the testing technique that is most frequently used to identify SARS-CoV-2. We have outlined the most recent developments in conventional medicines for the treatment of COVID-19 to be examined, including vaccination, antiviral medications, such as remdesivir, chloroquine or hydroxychloroquine, favipiravir, and anti-SARS-CoV-2 monoclonal antibody treatment. The broad range of treatment strategies works to determine the most effective action. This study's objective is to explain the diagnostic and therapeutic approaches applied to COVID-19 patients.
KEYWORDS: Antiviral drugs; Antiviral vaccines; Covid-19; Diagnostic methods
Download this article as:| Copy the following to cite this article: Keerthika S, Kowsalya M, Kameswaran R, Venkateswaramurthy N. Review of Covid-19's Current Development in Therapeutic and Diagnostic Techniques. Biosci Biotech Res Asia 2023;20(4). |
| Copy the following to cite this URL: Keerthika S, Kowsalya M, Kameswaran R, Venkateswaramurthy N. Review of Covid-19's Current Development in Therapeutic and Diagnostic Techniques. Biosci Biotech Res Asia 2023;20(4). Available from: https://bit.ly/3tCLBRM |
Introduction
The coronavirus-caused new pneumonia case was reported in Wuhan, a city in China, in December 2019. The virus was later identified and given the name COVID-19 (severe acute respiratory syndrome coronavirus, or SARS-CoV-2).1,2 On March 11, 2020, the World Health Organisation (WHO) declared COVID-19 a worldwide pandemic. Based on the WHO, at least 64,897,870 COVID-19 cases and 1,500,271 fatalities associated with it were reported globally as of December 3, 2020.3 The COVID-19 epidemic now poses the greatest threat to the world, according to the WHO. SARS-CoV-2 is one of three highly virulent coronaviruses that pose a global threat to public health, together with MERS-CoV and SARS-CoV-2.5 Fever, sore throat, exhaustion, coughing, and dyspnea are among the usual COVID-19 symptoms, which are also present when the infection is recent. Internationally, there have been fewer new confirmed and suspected cases as a result of interventions and control measures implemented by governments all over the world as well as changes in individual behavior (such as wearing masks & isolating oneself from social interactions).6 Although individuals of all ages have experienced severe lung damage, the virus is more likely to result in severe interstitial pneumonia, acute respiratory distress syndrome (ARDS), and subsequent multiorgan failure in some high-risk populations, such as the elderly or those who have multiple illnesses. These conditions are to blame for severe acute respiratory failure and high death rates. The degree of dyspnea and radiological indications that affected people often exhibit varies.7,8 As of right now, COVID-19 has no proven treatments. Therefore, managing COVID-19 patients required early identification, immediate patient isolation, and creation of protective circumstances to stop the infection.9 Real-time reverse transcription-PCR (RT-PCR) is the most popular test for identifying SARS-CoV-2.10 The accepted standard of care at the moment is supportive care, including ventilation, oxygenation, and fluid control. Antivirals can be used to treat SARS-COV-2 safely and efficiently. Currently, many treatments are being tested, including vaccination, and antiviral drugs such as remdesivir, favipiravir, monoclonal antibodies, and chloroquine/hydroxychloroquine.11
Diagnosis
The COVID-19 diagnosis is an important first step in locating the virus and understanding its epidemiology. The most critical element in making a COVID-19 diagnosis is the early identification of symptoms in clinical settings. The capacity to target and detect specific infections makes molecular techniques more suitable now for accurate diagnosis than syndromic testing and computed tomography (CT) scans.12 Since the SARS-CoV-2 infection was revealed in December 2019 for the diagnosis of COVID-19, several diagnostic tools and tests have been created. Reverse transcription-polymerase chain reaction (RT-PCR) techniques for viral RNA identification from clinical samples of SARS-CoV-2 infection are largely dominated by the COVID-19 test, which is already commercially available. The diagnosis of COVID-19 can also be made using other techniques, including chest computed tomography (CT) scans, immunological and serological testing for anti-SARS-CoV-2 antibodies, hybridization microarray assays, and isothermal nucleic acid amplification assays.13-15
RT-PCR test
The diagnostic procedure known as RT-PCR can utilize a bronchoalveolar lavage (BAL) sample, tracheal aspirate sample, or nasal swab sample. The main and most effective procedure for diagnosis is the collection of upper respiratory samples using nasopharyngeal and oropharyngeal swabs. As a COVID-19 diagnostic procedure, bronchoscopy is not recommended since the resulting aerosol poses a serious risk to patients and medical professionals. If upper respiratory samples are negative and additional diagnostic techniques would dramatically change the clinical course of treatment, only intubated patients could be examined for a bronchoscopy. However, when clinical and safety standards are met and a definite diagnosis cannot be made, bronchoscopy may be advised. Tracheal aspiration and non-bronchoscopic BAL (Bronchoalveolar Lavage) are other techniques for taking respiratory samples from intubated patients. Lavage of the bronchial tubes is also known as cleaning of the bronchial tubes. The BAL procedure is performed to obtain a lung sample for analysis. An airway wash and fluid sample are both obtained during the operation by passing a saline solution through the bronchoscope.14,16 Numerous studies have shown that blood and stool samples can contain SARS-CoV-2 RNA.17-20 The duration of SARS-CoV-2 RNA persistence in extrapulmonary organs, as well as in the upper and lower respiratory tracts, is uncertain. As in some cases of SARS-CoV-2 or MERS-CoV infection, viral RNA might continue to be detectable for weeks. All of the samples of blood, urine, stool, and lungs contained live SARS-CoV-2.21-29
Despite the possibility of sample contamination producing false-positive results, particularly in asymptomatic individuals, the RT-PCR test seems to have a very high level of specificity. It is unknown what the sensitivity rate is, however it is predicted to be between 66 and 80%. Even if there were no symptoms or other signs of infection, the positive percentage could be more than 50% if the person had been among others who were.30,31A single negative test may not exclude SARS-CoV2 infection when using nasopharyngeal swab material early in the epidemic, particularly in highly exposed patients. This circumstance can call for repeating the test or getting a BAL sample from the deeper respiratory system.
Serological test
Serological studies, which look for the presence of specific biomarkers like antibodies in blood serum or other biological fluids, are used to monitor the progression of disease. Several serological techniques, including the enzyme-linked immunosorbent assay (ELISA), have been examined to assess who has developed antibodies against SARS-CoV-2 virus infection. A lateral flow serological immunoassay called the qSARS-CoV-2 IgG/IgM rapid test was recently given FDA approval by Cellex Inc. Two distinct ELISA immunoassay types for the detection of COVID-19 antigens or antibodies to virus antigens have been developed. Although the microwell plate is coated differently in these tests, they are comparable. When the virus is coated with antigens, it is used to identify antibodies made against the virus. But coating them with antiviral antibodies makes it possible to recognize viral antigens.32-34
CT-Imaging test
A lack of testing kits led to the use of computed tomography (CT) scans as an early detection technique for COVID-19 in several states.[35] The diagnostic characteristic for COVID-19 was abnormal findings in the chest CT scan imaging. On chest CT scans, COVID-19 patients often displayed bilateral and peripheral ground-glass opacities in the early stages of the disease, while irregularly formed pavement patterns were apparent in the later stages.36,37
Treatment Strategies
There are currently no approved medications for the treatment of COVID-19 disease, nor is there a vaccination. Supportive therapy, symptom and treatment, and attempts to prevent respiratory failure make up the bulk of management.38-40 To prevent infection from spreading to other patients, family members, and healthcare professionals, it is crucial to guarantee patient isolation. Both symptomatic and asymptomatic infected people, as well as everyone who may have come into touch with them, must be quarantined.41 The amount of time spent outside and in social contact must be restricted for entire populations.42 The recommended course of action in moderate instances is self-isolation at home while keeping appropriate nourishment and hydration and treating symptoms like fever, sore throat, or cough. As a result, for serious instances, hospital beds may be available.43 Antibiotic prophylaxis is not effective in preventing bacterial superinfection, and there is also no proof that procalcitonin has a diagnostic function in COVID-19 patients.44 Anticoagulant medication is appropriate when the D-dimer result is four times above normal or the patient has early-stage COVID-19. Increased ischemic events and disseminated intravascular coagulation can occur when the coagulation system becomes overactive due to infection, inflammation, and other disease-related causes.45
Virus-Fighting Medication
A variety of antiviral medications have demonstrated their effectiveness in treating COVID-19 invitro, in animal models, and through case reports from human patients.46-52 Nearly all of this research is based on knowledge of the SARS-CoV-2 and MERS-CoV. However, antiviral medications should be avoided in those with comorbidity conditions, who have a higher mortality risk, or who have moderate to severe COVID-19 symptoms. Antihypertensive medications, antidiabetic medications, bronchodilators, thyroid hormones, and immunosuppressant medications were the most frequently given medications for comorbidities.4
Pharmacological Treatment
Remdesivir
In China, Remdesivir has been utilized successfully in many COVID-19 patients.46 The most promising results in antiviral therapy emerged with Remdesivir, an RNA-dependent polymerase inhibitor extensively studied for its potential to treat SARS-CoV-2 infection. Differing from other nucleotide analogs, remdesivir is a phosphoramidite prodrug that exhibits wide-ranging efficacy against various virus families, such as Filoviridae, Paramyxoviridae, Pneumoviridae, as well as Orthocoronavirinae encompassing severe acute respiratory syndrome and Middle East respiratory syndrome coronaviruses.53,54 Developed by Gilead Sciences in 2017 to combat Ebola virus infection, Remdesivir initially targeted this purpose. Subsequently, multiple phase 3 trials were initiated in the United States, South Korea, and China to evaluate its efficacy for both severe and moderate cases of illness. Recently, results from a double-blind, randomized, placebo-controlled trial investigating intravenous remdesivir in hospitalized Covid-19 patients displaying lower respiratory tract symptoms were published.55
The combination of lopinavir and ritonavir, second-generation antiretroviral drugs, functions by inhibiting viral protease. This combination has established pharmacological interactions and safety profiles, and it is easily accessible. The efficacy of lopinavir/ritonavir against SARS-CoV-2 has been demonstrated, and these medications also seem to reduce the viral load in individuals afflicted with COVID-19.49-51 Multiple randomized controlled trials are currently underway in China. The prescribed dose for lopinavir/ritonavir is 400/100 mg administered twice daily (BID), and there’s no need for adjustment based on glomerular filtration rate. Nonetheless, monitoring transaminases is often advisable. However, this drug combination is associated with several drug interactions and commonly leads to adverse effects such as nausea, diarrhea, and insomnia.
Chloroquine/Hydroxychloroquine
Chloroquine A tiny molecule medication of the aminoquinoline class called chloroquine is used to treat malaria. In addition, rheumatoid arthritis and HIV were both treated with chloroquine.56 This medication may have a broad spectrum of antiviral effects on all phases of viral entry and replication.57 A medication called hydroxychloroquine suppresses the immune system and is used to treat autoimmune diseases such as systemic lupus erythematosus, Sjogren’s syndrome, and rheumatoid arthritis. Since 1955, the FDA has authorized the use of hydroxychloroquine as a powerful antiparasitic medication.58,59 Hydroxychloroquine and chloroquine are currently being tested in clinical trials to ensure their efficacy in preventing SARS-CoV-2 infection.60 Another benefit of hydroxychloroquine is that it lowers the chance of thrombosis, which is a significant risk factor for SARS-CoV-2 patients. While hydroxychloroquine is a significant medication used both preventatively and directly to treat COVID-19 patients, several unfavorable side effects, such as cardiac arrest and ventricular arrhythmias, have lately been documented.[61-64]Furthermore, they seem to disrupt the ACE2 cell receptor and exhibit immunomodulatory properties, crucial for impeding the fusion of the virus and the host cell. This characteristic could elucidate their potent antiviral effects.52,65-70 For chloroquine and hydroxychloroquine, the recommended dosages are 500 mg BID and 200 mg BID, respectively. A loading dosage should be given before a maintenance dose for the best possible outcome.71 According to Yao et al., hydroxychloroquine is more effective than chloroquine at inhibiting SARS-CoV-2 in vitro.72 Typical side effects of these medications encompass nausea, vomiting, diarrhea, abdominal pain, headaches, as well as visual and extrapyramidal issues. Given its recognized potential for arrhythmogenic cardiotoxicity, diligent monitoring of blood counts and QT intervals is imperative.73
Favipiravir
Favipiravir is an antiviral medication of the pyrazine class that was primarily used in Japan to treat influenza.74,75 Its mechanism involves inhibiting the RNA-dependent RNA polymerase (RdRp) enzymes, which are crucial for the transcription and replication of viral genomes.76 In addition to its usage in influenza treatment, it underwent evaluations for addressing the Ebola virus, and more recently, it was subjected to assessments for potential application in treating COVID-19.77
Monoclonal Antibodies
Sarilumab/Tocilizumab
Sarilumab, known as Kevzara, is a humanized monoclonal antibody that targets the IL-6 receptor. Originally sanctioned by the FDA for treating rheumatoid arthritis, sarilumab is currently being investigated in clinical trials for its potential to treat severely ill COVID-19 patients afflicted with pneumonia. These trials explore its efficacy either as a standalone treatment or in conjunction with hydroxychloroquine, azithromycin, and/or corticosteroids.78,79 Humanized monoclonal antibody tocilizumab blocks the IL-6 signaling pathway by binding to both membrane-bound and soluble IL-6 receptors.80 When the SARS-CoV-2 virus attaches to alveolar epithelial cells and activates the innate and adaptive immune systems, a multitude of cytokines are released. These include IL-6, IL-10, and IL-23. Notably, IL-6 serves a dual role, functioning both as a pro-inflammatory and an anti-inflammatory cytokine.81-83 Tocilizumab is currently undergoing clinical trials, either as a standalone treatment or in combination with hydroxychloroquine, methylprednisolone, or azithromycin, for the management of SARS-CoV-2 patients. These trials are based on promising results observed in severely ill COVID-19 patients with pneumonia, indicating its potential effectiveness in treating such cases.
Paxlovid
Paxlovid made history as the first oral antiviral medication to gain approval in the United States for the treatment of COVID-19. Following suit, on December 23, 2021, Merck’s molnupiravir also received approval for the same purpose. In the case of Paxlovid, nirmatrelvir plays a crucial role by inhibiting the primary protease (Mopar) of SARS-CoV-2, thereby halting the virus’s replication. While ritonavir doesn’t directly affect SARS-CoV-2, it indirectly enhances nirmatrelvir’s effectiveness by preventing the metabolism of nirmatrelvir by CYP3A, leading to increased serum concentrations. Paxlovid is typically packaged with nirmatrelvir 150-mg tablets and ritonavir 100-mg tablets in cartons. The recommended dosage is two nirmatrelvir tablets along with one ritonavir tablet, taken twice daily for a five-day course, resulting in a 300/100 mg dose.
Vaccination
The U.S. Food and Drug Administration has revised the emergency use authorizations (EUAs) for the Moderna COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine. These changes now allow for the utilization of bivalent vaccine formulations as a sole booster dose, to be administered at least two months following the primary or booster vaccination.
Moderna COVID-19 Vaccines
The FDA’s announcement on January 31, 2022, marked the second approval of a COVID-19 vaccine. The vaccine, previously known as the Moderna COVID-19 Vaccine, is now marketed as Spikevax and is intended for the prevention of COVID-19 in individuals aged 18 and above. This monovalent vaccine is administered as a two-dose primary series.For individuals aged 18 and above, the recommended dose of the Moderna Vaccine is 50 µg (0.5 mL), with a cost ranging from $32 to $37. This vaccine provides immunity for at least 119 days after the initial vaccination and demonstrates a 94.5% efficacy in preventing SARS-CoV-2 infection. However, there have been isolated reports of associated adverse reactions.
Pfizer-BioNTech COVID-19 Vaccine
The FDA’s declaration on August 23, 2021, marked the initial endorsement of a COVID-19 vaccine, commercially named Comirnaty, formerly recognized as the Pfizer-BioNTech COVID-19 Vaccine. Its purpose is to safeguard individuals aged 12 and above from COVID-19. Subsequently, on February 11, 2022, the CDC, in partnership with the FDA, updated the guidelines for emergency use. These revisions encompassed comprehensive information about the primary, supplementary, and booster dosages of the Pfizer COVID-19 vaccines, specifically tailored to certain demographics.Individuals aged 16 and above are recommended to obtain the Pfizer-BioNTech Vaccine, comprising a 30 µg (0.3 mL) dosage at a cost of $19.50. This vaccine boasts a 95% efficacy in thwarting SARS-CoV-2 infection and confers immunity for a minimum of 119 days post the first inoculation. There have been isolated instances of associated unfavorable responses.
Table 1: COVID-19 Vaccination.
|
VACCINE |
TYPE |
AGE GROUP |
DOSE |
VACCINATION SCHEDULE FOR IMMUNIZATION |
EFFECTIVENESS |
|
Oxford Uni- AstraZeneca (Covishield) |
Viral vector |
18 years and older |
2 dose |
2 doses were separated by approximately 4 weeks. |
62-90% |
|
Moderna |
RNA |
18 years and older |
2 dose |
2 doses were separated by approximately 4 weeks. |
95% |
|
Pfizer-BioNTech |
RNA |
5-11 years & 12 years and older |
2 dose |
2 doses were separated by approximately 4 weeks. |
95% |
|
Gamaleya (Sputnik V) |
Viral vector |
18 years and older |
2 dose |
2 doses were separated by approximately 4 weeks. |
92% |
|
Janssen |
Viral vector |
From age 18 |
1 dose |
1 dose* |
72% |
|
Novavax |
Protein- based |
From age 18 |
2 dose |
2 doses were separated by approximately 4 weeks. |
90% |
|
Covaxin |
Inactivated |
18 years and older |
2 dose |
2 doses were separated by approximately 4 weeks. |
80.6% |
|
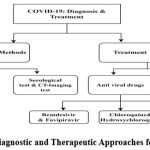
Figure 1: Diagnostic and Therapeutic Approaches for COVID-19
|
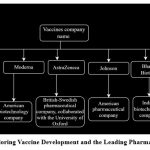 |
Figure 2: Title: Exploring Vaccine Development and the Leading Pharmaceutical Companies.
|
Conclusion
The SARS-CoV-2 virus is responsible for the ongoing COVID-19 pandemic, characterized by atypical pneumonia. The pandemic poses significant health risks, prompting the need for effective treatments. Nirmatrelvir and ritonavir, marketed as Paxlovid, have shown efficacy in managing COVID-19 symptoms and preventing SARS-CoV-2 infection. Additionally, mRNA boosters from Pfizer-BioNTech and Moderna are commonly administered. Utilizing advancements in technology, this knowledge can aid in refining vaccine and treatment development. To curb transmission until these solutions are widely accessible, early diagnosis via symptomatic and asymptomatic testing, contact tracing, quarantining, and supportive care are crucial for managing the pandemic effectively.
Acknowledgement
We would like to express our sincere gratitude to all the researchers and scientists whose work has contributed to our understanding of Review Of Covid-19’s Current Development In Therapeutic And Diagnostic Techniques. Their insights into the pathophysiology and therapeutic targets have been invaluable in the preparation of this review. We would also like to thank our colleagues and peers for their constructive feedback and support throughout the writing process.
Conflict Interest
There are no conflict of interest
Funding Sources
There is no funding sources.
References
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536-544.
CrossRef - Gralinski LE, Menachery VD. Return of the Coronavirus: 2019-nCoV. Viruses. 2020;12(2):135.
CrossRef - Sethi BA, Sethi A, Ali S, Aamir HS. Impact of the Coronavirus disease (COVID-19) pandemic on health professionals.Pak J Med Sci. 2020;36(COVID19-S4):S6-S11.
CrossRef - A, Jain M, Yadav R, Rathi P. Managing comorbidities in Covid-19 patients: A drug utilization study in a COVID-dedicated hospital in Northern India Sharma. J Family Med Prim Care. 2021;10(9):3387-3394.
CrossRef - Tang P, Wang J, Song Y. Characteristics and pregnancy outcomes of patients with severe pneumonia complicating pregnancy: a retrospective study of 12 cases and a literature review. BMC Pregnancy Childbirth. 2018;18(1):434.
CrossRef - Gavriatopoulou M, Ntanasis-Stathopoulos I, Korompoki E, et al. Emerging treatment strategies for COVID-19 infection. Clinical and Experimental Medicine.
- Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J Infect. 2020;80(6):e14-e18.
CrossRef - Lake MA. What we know so far: COVID-19 current clinical knowledge and research. Clin Med(Lond). 2020;20(2):124-127.
CrossRef - Majumder J, Minko T. Recent Developments on Therapeutic and Diagnostic Approaches for COVID-19. TheAAPS Journal. 2021;23(1).
CrossRef - LeBlanc JJ, Gubbay JB, Li Y, et al. Real-time PCR-based SARS-CoV-2 detection in Canadian laboratories. J ClinVirol. 2020;128:104433.
CrossRef - Gavriatopoulou M, Ntanasis-Stathopoulos I, Korompoki E, et al. Emerging treatment strategies for COVID-19 infection. Clinical and Experimental Medicine.
- Udugama B, Kadhiresan P, Kozlowski HN, et al. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano. 2020;14(4):3822-3835.
CrossRef - Kim H. Outbreak of novel coronavirus (COVID-19): What is the role of radiologists?. 2020;30(6):3266-3267.
CrossRef - Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323(18):1843-1844.
CrossRef - Carter LJ, Garner LV, Smoot JW, et al. Assay Techniques and Test Development for COVID-19 Diagnosis. ACS Cent Sci. 2020;6(5):591-605.
CrossRef - WHO, Laboratory testing for 2019 novel coronavirus (2019‐nCoV) in suspected human cases. Interim Guide. Geneva, Switzerland: World Health Organization site, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9(1):386-389.
CrossRef - Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
CrossRef - Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA. 2020;323(15):1488-1494.
CrossRef - Zhang Y, Chen C, Zhu S, et al. Isolation of 2019-to from a Stool Specimen of a Laboratory-Confirmed Case of the Coronavirus Disease 2019 (COVID-19). China CDC Wkly. 2020;2(8):123-124.
CrossRef - Memish ZA, Assiri AM, Al-Tawfiq JA. Middle East respiratory syndrome coronavirus (MERS-CoV) viral shedding in the respiratory tract: an observational analysis with infection control implications. Int J Infect Dis. 2014;29:307-308.
CrossRef - Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623.
CrossRef - Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015;386(9997):995-1007.
CrossRef - Chan KH, Poon LL, Cheng VC, et al. Detection of SARS coronavirus in patients with suspected SARS. Emerg Infect Dis. 2004;10(2):294-299.
CrossRef - Cheng PK, Wong DA, Tong LK, et al. Viral shedding patterns of coronavirus in patients with the probable severe acute respiratory syndrome. 2004;363(9422):1699-1700.
CrossRef - Hung IF, Cheng VC, Wu AK, et al. Viral loads in clinical specimens and SARS manifestations. Emerg Infect Dis. 2004;10(9):1550-1557.
CrossRef - Peiris JS, Chu CM, Cheng VC, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study.Lancet. 2003;361(9371):1767-1772.
CrossRef - Liu W, Tang F, Fontanet A, et al. Long-term SARS coronavirus excretion from the patient cohort, China. Emerg Infect Dis. 2004;10(10):1841-1843.
CrossRef - Corman VM, Albarrak AM, Omrani AS, et al. Viral Shedding and Antibody Response in 37 Patients With Middle East Respiratory Syndrome Coronavirus Infection. Clin Infect Dis. 2016;62(4):477-483.
CrossRef - Ai T, Yang Z, Hou H, et al. Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020;296(2):E32-E40.
CrossRef - Zhuang GH, Shen MW, Zeng LX, et al. Zhonghua Liu Xing Bing XueZaZhi. 2020;41(4):485-488.
- Nuccetelli M, Pieri M, Grelli S, et al. SARS-CoV-2 infection serology: a useful tool to overcome lockdown. Cell Death Discov. 2020;6:38.
CrossRef - Bryant JE, Azman AS, Ferrari MJ, et al. Serology for SARS-CoV-2: Apprehensions, opportunities, and the path forward. SciImmunol. 2020;5(47):eabc6347.
CrossRef - Li JX. Cellex qSARS-CoV-2 IgG/IgM Rapid Test. FDA, U.S. Food and Drug Administration.
- Ai T, Yang Z, Hou H, et al. Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases.Radiology. 2020;296(2):E32-E40.
CrossRef - Fu F, Lou J, Xi D, et al. Chest computed tomography findings of coronavirus disease 2019 (COVID-19) pneumonia. EurRadiol. 2020;30(10):5489-5498.
CrossRef - Lee EYP, Ng MY, Khong PL. COVID-19 pneumonia: what has CT taught us? Lancet Infect Dis. 2020;20(4):384-385.
CrossRef - Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1-9.
- Pang J, Wang MX, Ang IYH, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel Coronavirus (2019-nCoV): A Systematic Review. J Clin Med. 2020;9(3):623.
CrossRef - Shanmugaraj B, Malla A, Phoolcharoen W. Emergence of Novel Coronavirus 2019-nCoV: Need for Rapid Vaccine and Biologics Development. Pathogens. 2020;9(2):148.
CrossRef - Brooks SK, Webster RK, Smith LE, et al. The psychological impact of quarantine and how to reduce it: a rapid review of the evidence.Lancet. 2020;395(10227):912-920.
CrossRef - Parmet WE, Sinha MS. Covid-19 – The Law and Limits of Quarantine. N Engl J Med. 2020;382(15):e28.
CrossRef - Singhal T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J Pediatr. 2020;87(4):281-286.
CrossRef - Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846-848.
CrossRef - Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9(1):727-732.
CrossRef - Al-Tawfiq JA, Al-Homoud AH, Memish ZA. Remdesivir is a possible therapeutic option for COVID-19. Travel Med Infect Dis. 2020;34:101615.
CrossRef - Agostini ML, Andres EL, Sims AC, et al. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio. 2018;9(2):e00221-18.
CrossRef - Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11(1):222.
CrossRef - Chu CM, Cheng VC, Hung IF, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252-256.
CrossRef - Lim J, Jeon S, Shin HY, et al. Case of the Index Patient Who Caused Tertiar4y Transmission of COVID-19 Infection in Korea: the Application of Lopinavir/Ritonavir for the Treatment of COVID-19 Infected Pneumonia Monitored by Quantitative RT-PCR. J Korean Med Sci. 2020;35(6):e79.
CrossRef - Yao TT, Qian JD, Zhu WY, Wang Y, Wang GQ. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-A possible reference for coronavirus disease-19 treatment option. J Med Virol. 92020;92(6):556-563.
CrossRef - Han W, Quan B, Guo Y, et al. The course of clinical diagnosis and treatment of a case infected with coronavirus disease 2019. J Med Virol. 2020;92(5):461-463.
CrossRef - Sheahan TP, Sims AC, Graham RL, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. SciTransl Med. 2017;9(396):eaal3653.
CrossRef - Martinez MA. Compounds with Therapeutic Potential against Novel Respiratory 2019 Coronavirus. Antimicrob Agents Chemother. 2020;64(5):e00399-20.
CrossRef - Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the Treatment of Covid-19 – Preliminary Report. Reply. N Engl J Med. 2020;383(10):994.
CrossRef - Plantone D, Koudriavtseva T. Current and Future Use of Chloroquine and Hydroxychloroquine in Infectious, Immune, Neoplastic, and Neurological Diseases: A Mini-Review. Clin Drug Investig. 2018;38(8):653-671.
CrossRef - Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19?. Int J Antimicrob Agents. 2020;55(5):105938.
CrossRef - Satarker S, Ahuja T, Banerjee M, et al. Hydroxychloroquine in COVID-19: Potential Mechanism of Action Against SARS-CoV-2. CurrPharmacol Rep. 2020;6(5):203-211.
CrossRef - White NJ, Watson JA, Hoglund RM, Chan XHS, Cheah PY, Tarning J. COVID-19 prevention, and treatment: A critical analysis of chloroquine and hydroxychloroquine clinical pharmacology. PLoS Med. 2020;17(9):e1003252.
CrossRef - Centers for Disease Prevention and Control (CDC): Interim guidelines for collecting, handling, and testing clinical specimens from persons for coronavirus disease 2019 (COVID-19)
- Pal A, Pawar A, Goswami K, Sharma P, Prasad R. Hydroxychloroquine and Covid-19: A Cellular and Molecular Biology Based Update. Indian J ClinBiochem. 2020;35(3):274-284.
CrossRef - Offerhaus JA, Wilde AAM, Remme CA. Prophylactic (hydroxy)chloroquine in COVID-19: Potential relevance for cardiac arrhythmia risk. Heart Rhythm. 2020;17(9):1480-1486.
CrossRef - Zang Y, Han X, He M, Shi J, Li Y. Hydroxychloroquine use and progression or prognosis of COVID-19: a systematic review and meta-analysis. NaunynSchmiedebergs Arch Pharmacol. 2021;394(4):775-782.
CrossRef - Lei ZN, Wu ZX, Dong S, et al. Chloroquine and hydroxychloroquine in the treatment of malaria and repurposing in treating COVID-19. PharmacolTher. 2020;216:107672.
CrossRef - Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in the treatment of COVID-19-associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72-73.
CrossRef - Multicenter collaboration group of the Department of Science and Technology of Guangdong Province and Health Commission of Guangdong Province for chloroquine in the treatment of novel coronavirus pneumonia. ZhonghuaJie He He Hu Xi ZaZhi. 2020;43(3):185-188.
- Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279-283.
CrossRef - Touret F, de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020;177:104762.
CrossRef - Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949.
CrossRef - Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271.
CrossRef - Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55(4):105932.
CrossRef - Yao X, Ye F, Zhang M, et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020;71(15):732-739.
CrossRef - Haeusler IL, Chan XHS, Guérin PJ, White NJ. The arrhythmogenic cardiotoxicity of the quinoline and structurally related antimalarial drugs: a systematic review. BMC Med. 2018;16(1):200.
CrossRef - Shiraki K, Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. PharmacolTher. 2020;209:107512.
CrossRef - Venkataraman S, Prasad BVLS, Selvarajan R. RNA Dependent RNA Polymerases: Insights from Structure, Function, and Evolution. Viruses. 2018;10(2):76.
CrossRef - Shu B, Gong P. Structural basis of viral RNA-dependent RNA polymerase catalysis and translocation. Proc Natl AcadSci U S A. 2016;113(28): E4005-E4014.
CrossRef - Nagata T, Lefor AK, Hasegawa M, Ishii M. Favipiravir: a new medication for the Ebola virus disease pandemic. Disaster Med Public Health Prep. 2015;9(1):79-81.
CrossRef - Tu YF, Chien CS, Yarmishyn AA, et al. A Review of SARS-CoV-2 and the Ongoing Clinical Trials. Int J Mol Sci. 2020;21(7):2657.
CrossRef - Benucci M, Giannasi G, Cecchini P, et al. COVID-19 pneumonia treated with Sarilumab: A clinical series of eight patients. J Med Virol. 2020;92(11):2368-2370.
CrossRef - Masiá M, Fernández-González M, Padilla S, et al. Impact of interleukin-6 blockade with tocilizumab on SARS-CoV-2 viral kinetics and antibody responses in patients with COVID-19: A prospective cohort study. E Bio Medicine. 2020;60:102999.
CrossRef - Pereira MR, Aversa MM, Farr MA, et al. Tocilizumab for severe COVID-19 in solid organ transplant recipients: a matched cohort study. Am J Transplant. 2020;20(11):3198-3205.
CrossRef - Dastan F, Saffaei A, Haseli S, et al. Promising effects of tocilizumab in COVID-19: A non-controlled, prospective clinical trial. IntImmunopharmacol. 2020;88:106869.
CrossRef - Lipworth BJ, Chan R, Kuo CR. Tocilizumab for severe COVID-19 pneumonia. Lancet Rheumatol. 2020;2(11):660.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.