How to Cite | Publication History | PlumX Article Matrix
Aishwarya Savale1 , Rajendra Mogal1*
, Rajendra Mogal1* , Swati Talele1
, Swati Talele1 , Sumit Deore2
, Sumit Deore2 and Laxmikant Borse1
and Laxmikant Borse1
1Department of Pharmaceutics, Sandip Foundations, Sandip Institute of Pharmaceutical Sciences, Nashik, Maharashtra, India.
2Sandip University, School of Pharmaceutical Sciences, Nashik, Maharashtra, India.
Corresponding Author E-mail:rajendramogal85@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3168
ABSTRACT: Poor physicochemical properties like solubility, dissolution profile, stability, etc. of drugs remain as an obstacle to formulation development. In the last two decades, pharmaceutical cocrystal gaining lots of interest from researchers as these physicochemical, mechanical, and pharmacokinetic properties can be altered without compromising the therapeutic effect. The multi-component systems wherein one component is essentially the Active Pharmaceutical Ingredient and another may be either one more drug or coformer are termed as pharmaceutical cocrystal. Cocrystal is a novel, promising, and emerging approach to encountering such problems and improving the performance of pharmaceuticals. The present article describes the types, rationale of design, synthesis methods, and characterization of pharmaceutical cocrystals. In addition to this, challenges before and after synthesizing cocrystal along with currently approved formulations based on the pharmaceutical cocrystal approach and cocrystals under clinical investigation are highlighted.
KEYWORDS: Cambridge Structure Database; Hansen Solubility Parameter; Hydrogen Bonding Propensity; Pharmaceutical Cocrystal; Supramolecular Synthon
Download this article as:| Copy the following to cite this article: Savale A, Mogal R, Talele S, Deore S, Borse L. Pharmaceutical Cocrystals: A Novel Systematic Approach for the Administration of Existing Drugs in New Crystalline Form. Biotech Res Asia 2023;20(4). |
| Copy the following to cite this URL: Savale A, Mogal R, Talele S, Deore S, Borse L. Pharmaceutical Cocrystals: A Novel Systematic Approach for the Administration of Existing Drugs in New Crystalline Form. Biotech Res Asia 2023;20(4). Available from: https://bit.ly/47Aucb2 |
Introduction
Approximately 80 to 90 % of pharmacological compounds are accessible in solid form in the pharmaceutical industry, and the majority of them have poor solubility or belong to BCS classes II and IV. Accordingly, drug absorption in the gastrointestinal tract is reduced, pharmacological therapeutic applications are inhibited, and therapeutic product performance suffers1. Because of the drug’s weak biopharmaceutical qualities, it frequently fails in clinical development, resulting in considerable losses for the manufacturer.
To improve the solubility and other biopharmaceutical characteristics of drug molecules, many techniques have been used. The salt formation, solid dispersion, Nano-formulations, cosolvency, coamorphism, prodrug, size reduction, self-emulsifying formulations, and cocrystals are some of the methods used to modify the characteristics of pharmaceuticals. Multicomponent crystals for instance, salt, solvates, hydrates, and cocrystals have crucial part while developing new solid states, notably in the pharmaceutical sector. For example, in salt formation, both components are ionized and present in a single crystal lattice. In polymorphs, an active pharmaceutical ingredient (API) is present in a single lattice. While in cocrystals, neutral components, for instance API and coformer are linked together by non-ionic interactions. Coformer should be non-toxic and without adverse effect. As per United States Food and Drug Administration (USFDA), an ideal coformer would be “Everything added to food in the United State” (EAFUS). Approximately three thousand compounds are included that can be used as food additives or permitted as generally regarded as safe (GRAS)2.
A promising strategy to address issues related to pharmaceuticals is crystal engineering by cocrystallization3. Pharmaceutical cocrystals have offered so many opportunities to obtain the drug molecule possessing desired characteristics to enhance physical and chemical properties without varying the pharmacological activity of API’s. The cocrystallization method has also made it possible to create new non-ionic API solid forms with definite properties. A cocrystal differs from its parent crystals in terms of solubility, melting point, chemical stability, crystallinity, physical stability, and hygroscopicity. Using this procedure, one can alter an API’s properties to suit bioavailability requirements4.
Supramolecular chemistry is the base of cocrystal formation in which two or more molecules are present in a single cell bonded by hydrogen bonds, π-π interactions, or Van der Waals interactions. Drug’s intended qualities can be set by using a variety of synthons, coformers, and heterosynthon hierarchies3.
Quinhydrone was the first cocrystal discovered in 1844 by Wöhler, who discovered that combining benzoquinone solution with hydroquinone yields a crystalline molecule and is termed as ‘Quinhydrone’. The zigzag arrangement of chains made up of benzoquinone and hydroquinone molecules linked by O-H-O bonds was found to represent the geometry of cocrystals in 1958. Earlier, cocrystals were described as molecular compounds, heteromolecular complexes, addition compounds5, adducts, solid-state complexes6 before the name “cocrystal” was coined. In 1963, W. R. Lawton and E.F. Lopez used the term “cocrystal” for the first time to describe the crystalline complexes of organic amines and bisphenol.
A Comparison of the Usfda and European Medicines Agency (Ema) Pharmaceutical Cocrystals Conceptions
The United States Department of Health and Human services (HHM), the Centre for Drug Evaluation and Research (CDER), as well as the Food and Drug Administration (FDA), worked collectively to develop a comprehensive definition of cocrystals9. The FDA classified pharmaceutical cocrystals for the first time in April 20118. According to CDER cocrystals were classified as “drug product intermediate (DPI’s)” and also characterized as “solids that are crystalline materials consisting of two or more molecules in the same crystal lattice”8. However, this categorization was unable to meet the legal standards for cocrystals as DPI’s. Cocrystals defined by FDA as “crystalline material consisting of two or more molecules inside the same crystal lattice” using new regulatory classification in April 2013. In August 2016, the FDA again reclassified and defined the cocrystals as “crystalline material composed of two or more different molecules, typically drug and cocrystal formers (“coformers”)”10. After several revisions to defined the cocrystal, the FDA defined it as “crystalline material composed of two or more different molecules typically an API and cocrystal former (“coformer”) in the same crystal lattice”11.
EMA’s position on pharmaceutical cocrystals is significantly different from that of the FDA. In 2014, EMA issued a report on cocrystals, classifying them alongside salt. The regulations also state that cocrystals, like salts, are suitable for general application. A cocrystal must be different from API in terms of effectiveness and/or safety in order gain novel active substance (NAS) status. By comparing the therapeutic agent present at the site of pharmacological action with the approved product, the NAS status for various routes of administration will be established12.
Table 1: A comparison of the USFDA and EMAs pharmaceutical cocrystals conceptions78
|
USFDA |
EMA |
|
“Crystalline material composed of two or more different molecules, typically API and cocrystal formers (coformer), in the same crystal lattice”. |
“Solid that is crystalline single phase material composed of two or more different molecular and or ionic compounds generally in a stoichiometric ratio, which is neither solvates nor simple salts.” |
|
Cocrystal is referred as a new polymorph of API. |
Cocrystal referred similarly to the salt of the same API. |
|
According to USFDA API + drug or food grade coformers are the component of cocrystals. |
According to EMA, API + coformers in a fixed stoichiometric ratio form cocrystals. |
|
Regulatory status –DPI |
Regulatory status API |
|
Similar to the parent API |
Not similar (if their safety and efficacy are proved different) |
Types of Cocrystals
Researchers have looked at many types of cocrystals. Ionic cocrystals, molecular cocrystals, polymorphic cocrystals, solvate cocrystals, and hydrate cocrystals are some of the several types of cocrystals. Cocrystals can be classified as binary, tertiary, and quaternary on the basis of the number of constituents present in crystal lattice. Also be classified on the basis of the number of constituents present in the crystal lattice, such as binary, tertiary, and quaternary cocrystals.
Ionic cocrystals (ICC’s)
By ion-ion/ion-dipole forces and hydrogen bonding, the components in ICC’s structure interact. They have stronger ability to influence a molecule’s physicochemical properties than molecular cocrystals, which are essential for numerous applications, including pharmaceuticals, cosmetics, food, agrochemicals, and so on13. Ionic cocrystals are multicomponent crystal lattices in which the componenets are joined together by coordination bonds or hydrogen bonds, based on the presence of metal cations14. ICC comprises by crystallization of cation, anion, and neutral components. Conjugate acid-base cocrystals and acid salts are the major components of ICC. Cocrystallization of an organic drug molecule with an inorganic coformer can be used to modify the wide range of solid-statecharacteristics. The ionic cocrystals, however, are less thermodynamically stable than the drug’s salt form15,16 In 2010, the Braga group was the first to report the ICC, focusing on the solubility features of barbituric acid and the change in solubility characteristics with ICC synthesis with alkali bromides and caesium iodide17. The aqueous solubility of 6-mercaptopurine is considerably increased when an ionic cocrystal of the compound is created using zinc trifluoromethanesulfonate. By means of coordination bond and hydrogen bond, drug and coformer interact with each other18. Until now, the FDA has approved eight cocrystal formulations for commercial use, four of which can be characterized as ionic cocrystals; while others are molecular cocrystals19,15.
Molecular cocrystals (MCC’s)
MCC’s are crystalline solids contains two or more molecules in a definite stoichiometric ratio, viz. API and a pharmaceutically acceptable neutral compound or on the GRAS list82. When the term “molecular cocrystal” was not coined, it was described as “hydrogen bond complexes21,” “molecular organic compounds,”22, and “organic molecular compounds,”20 etc.
Solvated and hydrated cocrystals
The solvates wherein water molecules have been occluded into the crystal lattice are termed as hydrates. Because of small size of water molecules, existence of humidity in the air, and capability of water molecules to act as a hydrogen bond donor and acceptor, hydrates are more prevalent compare to other organic solvates. In solvates and hydrates, expansion of a cocrystal number of linked forms may be exploited23.
The thermal stability of cocrystal hydrates made up of isolated water molecules has been discovered to be greater than that of hydrates made up of water molecules in channels. According to thermal examinations, cocrystal hydrate was shown to be stable up to 150 °C in cocrystal hydrate screening of griseofulvin-acesulfame (artificial sweetener). The cocrystal hydrate exhibits higher solubility and dissolution rate over API, and it is stable at different relative humidity and temperatures24.
Types of Cocrystals on the Basis of the Number of Components in Crystal Lattice
Binary cocrystal’s
They are made up of two components, API and coformer, in a definite stoichiometric ratio in a single crystal lattice. Since the coformer is responsible for altering the physiochemical characteristic of the novel solid form, the drug’s pharmacological characteristics remain constant. To predict and handpicked appropriate coformers numerous strategies like Cambridge Structure Database (CSD), hydrogen bonding propensity (HBP), supramolecular synthon method, pKa rule, Hansen solubility parameter (HSP), phase diagram, etc. has been used. Binary cocrystals have a better chance of succeeding since just two separate components are engaged in interaction25. The intrinsic dissolution rate (IDR) and hygroscopicity of a binary cocrystal of naproxen and nicotinamide (2:1) were measured. The crystal structure and bond interactions between the API and the coformer were studied using single X-ray analysis and NMR studies. The cocrystal shows a good dissolution rate along with reduction in hygroscopicity over pure is API 26. Using both solid-state and solution-based techniques, cocrystals of indomethacine: saccharine (GRAS sweetener) (1:1) were produced. In cocrystal, indomethacine forms acid dimer synthon, whereas saccharin produces imide dimer synthon, with N-H-O hydrogen bonding holding both dimers together. In comparison to pure indomethacine, the cocrystal has a higher dissolution rate27. Similarly, following crystal engineering with malonic acid, famotidine demonstrates an increase in solubility without affecting stability. The amide group of famotidine undergoes intermolecular hydrogen bonding with the carboxylic group of malonic acid28. Some other examples of binary cocrystals are fluoxetine hydrochloride: succinic acid (2:1) cocrystal29, caffeine: oxalic acid cocrystal (2:1)30.
Ternary and Quaternary cocrystals
Three neutral solid-state molecules are connected to one another in a definite stoichiometric ratio in the crystal structure of a ternary cocrystal by hydrogen and/or halogen bonds31. The exact and balanced intermolecular interactions between the three components are essential for the effective design and development of ternary cocrystals25.
Relatively, fewer ternary or higher cocrystals have been reported than binary cocrystals, likely because it is not easy to stoichiometrically incorporate three or more coformers into a single cocrystal (Table 2).32 Therefore, formulation and development of ternary or higher cocrystal is a tough field of research. The design and development of ternary cocrystals has exceptional intellectual and artistic potential, however researchers have yet to investigate the functional uses of ternary and quaternary cocrystals33.
Table 2: Examples of ternary and quaternary cocrystals
|
|
Examples |
Reference |
|
Ternary cocrystals |
Isoniazid: Fumaric acid: nicotinamide(1:1:1) |
33 |
|
Isoniazid: Succinic acid: nicotinamide(1:1:1) |
33 |
|
|
3,5-dinitrobenzoic acid: isonicotinamide: 4-(N, N-dimethyl) aminobenzoic acid (1:1:1) |
34 |
|
|
Isoniazid –fumaric acid-pyrazinamide |
35 |
|
|
Quaternary cocrystals |
1,3cis,5cis-cyclohexanetricarboxylic acid-4,4’-bipyridine bases |
36 |
|
Isoniazide-2,5-dihydroxybenzoic acid- 2,4-dihydroxycinnamic acid- water molecule |
32 |
Polymorphic cocrytals
The ability of a substance to exist in two or more than two crystalline forms is termed as polymorphism37. Polymorphic behaviour of cocrystal is equally essential as single cocrystal38. Different types of polymorphic cocrystals can be classified based on synthon (synthon polymorphs), crystal forms and molecular arrangement (packing polymorphs, tautomeric polymorphs, conformational polymorphs)25. Polymorphism is referred as packing polymorphism when similar chemical moieties fit into overall 3D crystal packing or different packing of component molecules having equivalent conformations23.
In Synthon polymorphs, different primary synthons are present and possess different primary hydrogen bond structure. Synthon polymorphs are commonly found in molecules that have potential to develop different hydrogen bonding39.Supramolecular synthon that can be built by known intermolecular interactions have been defined using the synthon principles of organic synthesis. The phrase homomeric interaction, which also refers to homosynthons, is used to describe interactions between amides, carboxylic acids, pyridines, and oximes. In comparison to homosynthons, heterosynthons are more common in cocrystals like carboxylic acid-amide, carboxylic acid-pyridine, and alcohol-pyridine23.
Tautomeric polymorphism is the concomitance of crystalline tautomers in equilibrium conditions23.Various tautomers of a material crystallise in distinct crystal forms in the scenario of tautomeric polymorphism. Tautomeric polymorphs are formed when various tautomers of an organic molecule crystallise and coexist in equilibrium in many crystal forms. Tautomeric polymorphism arises when isomers are in a state of dynamic equilibrium with one another. The crystal forms that make up tautomers that interconvert in solution are known as polymorphs because they are thought of as being the same chemical entity. Tautomeric cocrystallization is a very infrequent occurrence39.
Conformational polymorphism is a type of polymorphism defined by molecular moieties having varying degrees of rotational freedom in the unit cell40. In the instance of conformational polymorphism, the compound’s molecules are organised in distinct molecular conformations. Conformational polymorphism has been attributed to flexibility of pharmaceutical molecule with large degrees of torsional flexibility. Pharmaceutical entities that are conformationally flexible may have a higher likelihood of exhibiting polymorphism because the energies required for rational bonding are alike to the lattice energy gap between polymorphs. In terms of cocrystals, many cocrystal polymorphs analysed display distinct coformers of the cocrystal ingredients and may therefore be classified as conformational cocrystal polymorphs5. Some examples of polymorphic cocrystals are listed in table3.
Table 3: Examples of polymorphic cocrystals
|
Types |
Examples |
Reference |
|
Synthon polymorphism cocrystal |
4-hydroxy benzoic acid:2,3,5,6-tetra-methylpyrazine (2:1) |
41 |
|
4-hydroxy benzoic acid: 4,4’-bipyridine (2:1) |
39 |
|
|
Caffeine: Glutaric acid (1:1) |
42 |
|
|
5-fluorouracil: 4-hydroxybenzoic acid (1:1) |
41 |
|
|
Tautomeric polymorphism cocrystal |
Piroxicam: 4-hydroxybenzoic acid |
43 |
|
Conformational polymorphism cocrystal |
Pimelic acid: 4,4’-bipyridine |
40 |
|
Nicotinamide: pimelic acid |
5 |
|
|
Packing polymorphism cocrystal |
Benzoic acid: 2-aminopyrimidine (2:1) |
44 |
|
Salicylic acid: N, N’-diacetylpiperazine
|
44 |
Methods and Tools for Rational Design of Pharmaceutical Cocrystals
The selection of an appropriate coformer is a critical step in the production of a novel cocrystal. Because the coformer is responsible for the process of improving poor drug qualities, selecting a proper and the most suitable coformer is the first step toward the production of a successful novel cocrystal with excellent physiochemical properties. There are several methods for selecting rational cocrystallizing API-coformer pairs to generate rational cocrystals, but they come at the expense of time and resources. As a result, there is a need for swift, low-cost, and time-saving virtual screening procedures for selecting an optimal cocrystallizing pair. There are many virtual screening methodologies that have been thoroughly established in literature studies that allow for the pre-screening of relevant coformers before the practical cocrystallization process45. Some of the main predictive tools for the development of pharmaceutical cocrystals include the CSD46, HSP47, phase diagram48, HBP, rule, Fabians methods, Conductor-like Screening Model for Real Solvents (COSMO-RS), cocktail cocrystal method2, supramolecular synthon approach.
Computational prediction of thermal stability of cocrystals has been studied by measuring crystal lattice energy through crystal structure prediction (CSP) to examine whether the cocrystal is thermodynamically more stable than pure API64.
CSD
It is a computational tool that can reduce experimental costs and generate libraries of appropriate coformers by providing valuable information regarding the molecular association of drugs and coformers based on functional groups involved in supramolecular synthesis via statistical analysis of packing motifs2. Statistical analysis of cocrystal data on the CSD enables to use virtual screening approaches to discover acceptable cocrystal forming pairings and allow cocrystal to be built using molecular modelling, reducing cost as well as time. CSD allows better understanding of the behaviour of molecules and intermolecular forces within a crystal, for the retrieval, display, and analysis of experimentally collected crystallographic data. This gives information on the type of intermolecular interaction, directional properties, geometrical preferences, and kinds of supramolecular synthon involved3.
HSP
On the basis of the compound’s structure, HSP may be used to assess the miscibility of API and coformer50. The fundamental unit of HSPs is the total energy of liquid vaporization, which is composed of numerous unique component forces. The difference between the API and coformer’s total solubility parameters (t) is calculated for the purpose of cocrystal formation prediction51. The Fedors technique, van Krevelen’s approach, and Hoy’s approach are the three group contribution approaches that are most frequently employed in the evaluation of HSP52,50. Greenhalgh and Krevelen offer a theoretical forecast for the production of cocrystals. According to krevelen if the difference in the associate’s solubility parameter values is less than 5MPa1/2, cocrystals may form while Greenhalgh recommends the formation of cocrystals if the difference is less than 7MPa1/2. Furthermore, Salem et al. recently submitted a cut-off value of 8.18MPa1/2, which is more trustworthy than prior values owing to the relaxation of the cut-off value50,53.
HBP
The cocrystal formation is promoted by hydrogen bonding, which is responsible for their synthesis. The API’s functional groups will interact with the coformer’s functional groups, and they will interact with each other. Carboxylic acids, amides, and alcohols are some of the frequently occurring functional groups of APIs and coformers that participate in hydrogen bonding54. In hydrogen binding, all excellent proton donors and acceptors are employed, and six-member ring intermolecular hydrogen bonds are preferred over intermolecular hydrogen bonding. After intermolecular hydrogen bond development, the best proton donor and acceptor create intermolecular hydrogen bonds with one another55.
Rule
Although it is unknown how salts and cocrystals differ from one another, it is possible to do so by utilizing the position of the proton in an acid and a base. The carboxyl proton is moved to the hydrogen of the base in salts, whereas the proton stays in the carboxyl group of acids in cocrystals56. One can predict the formation of salts or cocrystals based on transfer of a proton between acid and the base. A base’s and an acid’s values can be used to anticipate the development of salt or crystals. Formation of salt take place between acid and base if the value of [ is more than 2 or 3. Cocrystals almost usually form at lower values of (<0), whereas intermediate values of (0-3) cannot differentiate between cocrystals and salts3,13.
Supramolecular synthon approach
Drug molecules contain COOH, CONH2, OH, NH2, SONH2, and certain hydrogen bonding groups that allows crystallization to be regulated by supramolecular synthon51. Supramolecular synthons includes homosynthons and heterosynthons. In supramolecular homosynthons, two like complementary functional groups form crystalline complexes (e.g., carboxylic acid-acid dimers, and amide-amide dimers), whereas, in heterosynthons, two different complementary functional groups form the crystalline complexes. (e.g. Acid-amide, hydroxyl-amine, hydroxyl-pyridine, acid-pyridine)57. The probability of hydrogen bond formation can be determined when crystalline formations contain at least one hydrogen donor and acceptor. The potential to generate supramolecular homosynthons is as; Amide> acids>alcohols>58.
Phase diagram
The thermodynamic interactions between cocrystals and their discrete components can be described most frequently by phase diagrams. They can be used as a tool for searching more efficient cocrystal and predicting the best preparation method. Three types of phase diagrams have been used to depict phase behavior of crystallizing system viz. phase solubility diagram (PSD), Binary phase diagram (BPD), and ternary phase diagram (TPD)48. Binarized phase diagrams, also known as temperature composition maps, display the equilibrium phases that are present at a given temperature and API-coformer composition. The BPDs of an API and a coformer govern the cocrystal system’s thermal stability and whether or not there is sufficient contact between the two parts to produce a thermodynamically stable cocrystal48. Ternary phase diagrams are important for constructing crystallization processes, as well as for other screening approaches. It is a critical step to select the appropriate solvent for both screening and crystallization techniques59.TPD is a triangle phase diagram that shows the relationship between the solute-solute-solvent. It is used in solution cocrystallization for coformer screening12.
COSMO-RS
It is equilibrium thermodynamics software based on quantum chemistry that is often used to predict thermodynamic equilibrium characteristics in a liquid system. The excess (or mixing) enthalpy, ΔHex, of a virtual supercooled liquid of drug-coformer mixtures is utilised to forecast the affinity between molecules based on COSMO-RS theory. The excess enthalpy can be used as a variable to evaluate the tendency of those molecules to form a cocrystal, assuming that the interactions in the cocrystal are the same as in the liquid mixture. To be more specific, the lower the ΔHex, the more the molecule prefer the mixture concerning its pure form. The formation of cocrystal is more favourable when ΔHex is negative. On the other hand, lower the drug-coformer affinity if the value of ΔHex is higher. In such situation, the more negative the excess enthalpy, the more likely it is that the two molecules will form a cocrystal or co-amorphous state60.
Advanced Methods to Design Cocrystals
Hot melt extrusion (HME)
In this method, the raw materials are squeezed through a die with rotating screw at elevated temperature to produce a uniformly shaped product. Before proving to be viable process for the preparation of pharmaceutical dosage forms, HME was first used in the plastic and food industries26.HME is a relatively new method for producing high-quality cocrystals. HME is recognized as an environmentally friendly green technology because it can be used for continuous manufacturing, solvents are not required for the process, and can easily be scaled up without reducing the quality of the cocrystals. As a result, HME may lower the production cost, minimize waste and footprint, improve equipment performance, eliminate human intervention, and shorten the time it takes for the complete product to reach market9. Regardless of the fact that HME has been shown to be a cost-effective and scalable technique for cocrystal production, its main disadvantage is the development of significant shear stress during the processing of dry, stiff crystalline materials, which increases energy consumption and hastens extruder wear. A recently developed method called matrix-assisted cocrystallization (MAC), which includes processing drugs and coformer together in the presence polymeric matrix can prevent this. The melted polymer matrix improves the dissolving profile of pharmaceuticals, powder flow, and compressibility, as well as facilitates intimate mixing by decreasing excessive shear stresses61.
It is possible to prepare a product that satisfies the fundamental design requirements, such as crystallinity, physicochemical stability, compressibility, or dissolution rate, by varying process parameters such as the barrel temperature, screw configuration, screw speed and feed rate62. With caffeine and AMG 517 as a model, medina et al. Employed HME to show cocrystal synthesis26. They proved that twin-screw (TSE) may manufacture cocrystals without utilizing solvent by providing adequate surface contact among the cocrystal component owing to very efficient mixing and compact material packing63. Dhumalet et al. investigated the ibuprofen nicotinamide cocrystallization using solvent-free continuous cocrystallization (SFCC), which involves single-step cocrystallization and simultaneous agglomeration at a temperature approaching the lower melting components melting point or the eutectic temperature. The relevance of several processing factors like screw speed, screw design, and extrusion temperature) in HME cocrystal production was also underlined in this work64. As both the drug and the coformer must be miscible in molten form, this approach is not applicable for thermo -labile substances.
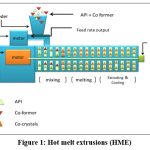 |
Figure 1: Hot melt extrusions (HME)
|
High shear granulation
This standard technique comprises agglomeration of small particles using granulating fluid in order to improve flowability, improve drug distribution, minimize segregation, and reduce exposure of drug to the surrounding. Rehder et al. investigated high-shear granulation techniques in which piracetam and tartaric acid cocrystals are granulated using calcium hydrogen phosphate unhydrous (CaHPO4), hydroxypropyl cellulose (HPC), and microcrystalline cellulose (MCC). High-shear granulation may become a practical method for preparing granules of cocrystal on a batch basis. In high-shear granulation, formation of cocrystal is influenced by the various factors like amount of granulation liquid incorporated, speed of impeller, and the additives used in formulation. However, the creation of cocrystals during high-shear wet granulation can help pharmaceutical manufacturers by facilitating downstream processing. Unintentional and the undesirable cocrystal formation must be avoided since it might effect compactibility and drug release, and consequently drug performance65.
Spray drying
In this method, the drug and the coformers are dissolved in a solvent to produce a suspension or solution. The resultant mixture is then sprayed with a hot air stream. When a solution comes incontact with heated air steam, it evaporates and transforms into solid particles known as cocrystals. The primary disadvantage of this technique is the low yield of cocrystals50. Alhalaweh et al. used spray drying to make highly crystalline cocrystals of theophylline from urea and saccharine as coformers. The author used nicotinamide and experimented with different solvent and solution concentrations before drying with nitrogen gas66.
Supercritical carbon dioxide processing
Supercritical fluids (SCF’s) are materials that have a temperature and pressure higher than their critical points and have features that are between a vapor and a liquid. That is, their density is equivalent to that of a liquid, permitting significant salvation power, but their viscosity and diffusivity are equivalent to those of vapor, allowing for efficient mass transfer50,67. Carbon dioxide (CO2) is the most commonly used supercritical fluid due to its nontoxic, non-flammable nature as well as its affordability.
This method involves mixing drug and conformer using magnetic stirrer in a supercritical CO2 pressurized vessel, and rapid expansion of the CO2 by gradually lowering the temperature results in cocrystal formation50. Due to the prompt drying of the droplets generated during the depressurization of the supercritical mixture, the solvent quickly evaporated, and the cocrystals precipitated in an appropriate stoichiometric ratio based on the prior molar ratio of the cocrystal components. The high density and low viscosity of supercritical fluids (SCF) lead to a significant increase in the liquid momentum and interfacial shear forces and hence upon depressurization, it transform into fine droplet by liquid jet68.
Anti-solvent cocrystallization (vapor diffusion)
The limited solubility of many API’s in supercritical CO2 and the excellent miscibility of supercritical CO2 in organic solvents are the basis of the anti-solvent technique. This method is based on the precipitation of a solute in an organic solvent when supercritical CO2 is added. The addition of supercritical CO2 lowers the solvent’s capacity to solvate API molecules, lowering API solubility. This results in API supersaturation and precipitation. Because of the fast phase change caused by simultaneous nucleation, crystal growth, and agglomeration, the crystalline phase is finally produced 69.
Carbamazepine-saccharine cocrystals were prepared by an antisolvent approach and were proven to be a suitable way of producing it’s cocrystals with high efficacy70.
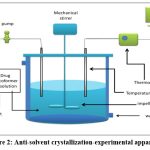 |
Figure 2: Anti-solvent crystallization-experimental apparatus
|
Freeze drying (lyophilisation)
During the freeze-drying procedure, the damp substance freezes, and then ice sublimates straight to vapor by using a low partial pressure of water vapor. As a cocrystallization technique, freeze drying enables the formation of a solid, amorphous mixture of two coformers from which a cocrystal can form without encountering the kinetic barrier of the crystalline seed of the two coformers71.
Microfluidic and jet dispensing approaches
With thousands of samples being screened per second and fluid networks being controlled by micrometer-sized channels, microfluidics technique is an effective method for high-throughput analysis9. In this technique, a supersaturated solution of API and coformer in several solvents is prepared in a very small quantity via combinatorial mixing. After that, the API is placed in a vertical microfluidic chip while the coformer is placed in a horizontal microfluidic chip. A two-step screening approach can be used to treat API with various coformers and solvents to identify the combinations that have the greatest tendency to form cocrystals72. This method was first presented by Goyalet al. (2012), who used a two-phase screening strategy to treat caffeine with a variety of coformers and solvents in order to identify the combinations that had the highest tendency for cocrystals72.
Ultrasound-assisted crystallization (sonocrystallization)
Cocrystals in the presence of ultrasound are thought to have a significant impact on crystallization. Ultrasound has been observed to have a positive influence on crystallization processes, with cocrystal components exhibiting a significant decrease in induction time in the presence of ultrasound73. This method involves dissolving the API and coformer in a suitable solvent. During sonication process, cold water is fed through the sonicator for maintaining the temperature steady and preventing fragmentation. Crystalline material melts, combines, and rapidly recrystallizes after cooling due to the energy sent to the sample during irradiation results in rapid rise in temperature within a short period. The coformer material should meet certain requirements, one of which is that it should be sublimable in order to aid in a nucleation process during the vapor phase3.
Mechanochemical Grinding
This process can be carried out either by neat grinding or liquid-assisted grinding (LAG). LAG is superior over other classic procedures such as slow evaporation and slurry-based approaches for cocrystallization in a range of conditions when solid-state grinding has failed 74. The basic principle of this method is kinetic energy-induced cocrystallization and can be done either by manual grinding or by mechanical grinding using ball milling. The components are introduced into steel balls where they undergo impaction, resulting in a reduction in particle size48. Mithu et al. synthesized a ketoconazole-dicarboxylic acids (fumaric acid and succinic acid) cocrystals to evaluate the effects of different grinding processes. They found that cocrystals are formed via liquid-assisted grinding (LAG) rather than neat grinding74. Other example is norfloxacin-nicotinic acid cocrystals which are efficiently synthesized via a mechanochemical approach75.
However, because this process involves separate grinding of the drug with the coformer, it is time-consuming. This is the main downside of this technique. The “cocrystal cocktail approach” can be used to get around this limitation. Yamamoto et al. introduced this synthon-based technique. In ball milling, multiple coformers with equivalent characteristics can be introduced with the drug. Several types of API-coformer pairings can be discovered concurrently, and their bonding hierarchies can be easily recognized, reducing workload76.
Reaction cocrystallization
This method is suitable when components of cocrystal exhibit different solubility. When cocrystal supersaturated solutions are created when reactants with non-stoichiometric concentrations are mixed, leading to cocrystal precipitation1. The cocrystal’s growth and nucleation are governed by the tendency of reactants to make it less soluble. Examples of cocrystals produced by reaction cocrystallization are; carbamazepine-saccharine cocrystal77, indomethacine-saccharine cocrystal78, posaconazole-4-aminobenzoic Acid79. Saturated coformer solutions are used in reaction cocrystallization in which a quantity of drug with a high solubility is added (Fig. 3 and 4). The API and coformer can be dissolved in a pure solvent, a solvent with solid ingredients, or by combining two previously dissolved solutions that contain the drug and coformer. A drug will dissolve in a saturated coformer solution up to its solubility limit and then precipitate cocrystals from the solution. The solution or slurry is agitated for sufficient time required to complete reaction and the filtered. Because pharmaceutical companies use stirred tank reactors for solution crystallization, it is a practical commercial alternative80.
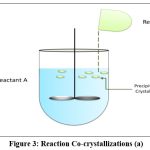 |
Figure 3: Reaction Co-crystallizations (a).
|
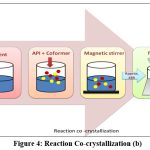 |
Figure 4: Reaction Co-crystallizations (b).
|
Characterization of Cocrystals
Synthesized cocrystals are characterized by numerous techniques viz. single crystal X-ray diffraction (SXRD), Fourier transform infrared spectroscopy (FTIR), powder X-ray diffraction (PXRD), Raman spectroscopy, differential scanning calorimetry (DSC), solid-state nuclear magnetic resonance spectroscopy (ssNMR), and scanning electron microscopy (SEM) are commonly used.
Extreme vibrations in the vibrational bands can be revealed by FTIR of cocrystals, crystals, and their polymorphs, as well as hydrates/solvates. One of the simplest ways to distinguish between polymorphs and cocrystals is through the use of vibrational spectroscopy. If specific bands or functional groupings are sensitive to a specific solid shape, it becomes much more significant23. ssNMR provides information on the chemical environment of organic nuclei, can be used to get structural information. Hydrogen bonding and chemical structure are revealed via Raman spectroscopy7. XRPD and ssNMR have been used in various research to solve crystal structures 81. Thermal analysis techniques such as DSC and thermal gravimetry (TG) can be used to measure melting temperature, crystallinity, solvate/hydrate formation, and the enthalpy of fusion. The melting temperature of cocrystals may differ from the original API temperature.
Thermal scans with temperature-controlled PXRD or concurrent DSC-PXRD systems reveal actual temperature changes that take place while being measured82. SEM is a kind of electron microscope that uses a strong electron beam to scan a material. Signals that reveal the sample’s surface topography are created when electrons contact with the atoms in the sample83.
The stability and therapeutic efficacy of cocrystals are similarly impacted by crystallinity. PXRD and DSC are frequently employed to determine the number of targeted cocrystals. To assess the crystallinity of carbamazepine-saccharin and indomethacin-saccharin cocrystals, Padrela et al. employed diffraction peak intensities specific to the target cocrystal in PXRD patterns and cocrystal melting enthalpy84. For complicated solid formulations, reliable crystallinity assessments to increase the use of pharmaceutical cocrystals might be difficult82.
Cocrystal Challenges
The major challenge in development of cocrystals is its instability like dissociation of conformer during formulation development, substitution of conformer, alteration in stoichiometric ratio, and during dissolution more soluble cocrystals have tendency to convert into the less soluble parent drug crystal. The inability to produce in vitro-in vivo correlation (IVIVC), scale-up, and polymorphism are all noteworthy obstacles to convert cocrystals into therapeutics. Cocrystal stability may be negatively impacted by the numerous excipients included in formulations. In solid-state formulations, water-mediated cocrystal dissociation has been reported85. Cocrystal dissociation is more likely to happen during operations that involve the addition of water, such as wet granulation or wet milling86. It was suggested that one strategy to reduce the risk of excipient-induced cocrystal dissociation was to remove an anion that would absorb protons from acidic coformers and metal cations that would react with acidic coformer anion85.
Some cocrystal formulation excipients may take the place of the original coformer during storage. As a result of coformer removal or alteration, original API crystals, API hydrate crystals, or other crystals with low solubility or bioavailability might develop82.
The second issue might be the transformation of a cocrystal with a higher solubility into the original, less soluble drug. While the cocrystal is dissolving, this can also happen. Surfactants, polymers, or a combination of the two may be used to avoid this7. Furthermore, cocrystal scale-up is a significant difficulty that might obstruct cocrystal development. Finally, establishing an IVIVC for cocrystal might be problematic because to the varying circumstances along the gastrointestinal system87.
Examples of Marketed and In-Clinical Trial Cocrystals
Table 4:Examples of marketed and in-clinical trial cocrystals88, 90,12,19,7
|
Cocrystal components |
Trade name |
FDA approval date/status |
Use |
|
Choral hydrate – betaine |
Beta-chlor® |
1963 |
Sedation |
|
Valporic acid – valporate sodium |
Depakote® |
1983 |
Epilepsy |
|
Caffeine – citric acid |
Cafcit® |
1999 |
Infantile apnoea |
|
Escitalopram oxalate – oxalic acid |
Lexapro ® |
2002 |
Depression |
|
Ipragliflozin – L-proline |
Sulgut® |
2014 |
Diabetes |
|
Valsartan sodium – sacubitril sodium |
Entresto® |
2015 |
Heart failure |
|
Sonidegib monophosphate – phosphoric acid |
Odomzo® |
2015 |
Basal cell carcinoma |
|
Ertugliflozin – pyroglutamic acid |
Steglatro® |
2017 |
Diabetes |
|
Aripiprazole – fumaric acid |
Abilify® |
2002 |
Schizophrenia |
|
Fluoxetine hydrochloride |
Prozac® |
1999 |
Depression |
|
Itraconazole |
Sporanox® |
2001 |
Antifungal |
|
Sildenafil citrate |
Viagra® |
1998 |
Erectile dysfunction |
|
Gentisic acid |
TAK-020 |
Phase I |
Bruton tyrosine kinase inhibitor |
|
Tramadol hydrochloride – celecoxib |
E-58425 |
phase III |
NSAID |
|
Zoledronic acid |
T 121E01F/T121E02F |
phase III |
Anticancer |
|
|
CC-31244 |
phase II a |
Non-nucleoside polymerase inhibitor |
Conclusion
Because cocrystal overcomes weak pharmacological qualities of drug molecules, it is a very promising strategy to enhance the biopharmaceutical aspects of old drugs. Regulatory agencies such as the USFDA and EMA have already released guidelines for cocrystals, and hence, cocrystals are gaining the interest of researchers. As a result of the rising use of cocrystals in drug development, a variety of advanced methods with significant advantages over conventional methods have been established hence the acceptance of cocrystals has increased.
Acknowledgment
The authors are thankful to Management, institute for providing necessary facilities, constant support, and encouragement to write this review article.
Conflict of Interest
There is no conflict of Interest.
Funding Source
There are no funding sources.
References
- Guo M., Sun X., Chen J., Cai T. Pharmaceutical cocrystals: A review of preparations, physicochemical properties and applications. Acta Pharm. Sin. B. 2021;11(8): 2537–64.
- Kumar S., Nanda A. Pharmaceutical cocrystals: An overview. Indian J Pharm Sci. 2017;79(6): 858–71.
- Buddhadev S.S., Garala K.C. Pharmaceutical Cocrystals—A Review. Proceedings. 2021;62(1): 14.
- Shikhar A., Bommana M.M., Gupta S.S., Squillante E. Formulation development of Carbamazepine-Nicotinamide cocrystals complexed with γ-cyclodextrin using supercritical fluid process. J Supercrit Fluids. 2011; 55(3): 1070–8.
- Aitipamula S., Tan R.B.H. Pharmaceutical cocrystals: Crystal engineering and applications. In: Multi-Component Crystals. 2017: 1–31.
- Karagianni A., Malamatari M., Kachrimanis K. Pharmaceutical cocrystals: New solid phase modification approaches for the formulation of APIs. Pharmaceutics. 2018; 10(1): 18.
- Panzade P.S., Shendarkar G.R. Pharmaceutical cocrystal: a game changing approach for the administration of old drugs in new crystalline form. Vol. 46, Drug Dev. Ind. Pharm. 2020; 46(10): 1559–68.
- Garg U., Azim Y. Challenges and opportunities of pharmaceutical cocrystals: A focused review on non-steroidal anti-inflammatory drugs. RSC Medicinal Chemistry. 2021;12(5): 705–21.
- Douroumis D., Ross S.A., Nokhodchi A. Advanced methodologies for cocrystal synthesis. Adv. Drug Deliv. Rev. 2017;117:178–95.
- Food and Drug Administration. Regulatory Classification of Pharmaceutical Cocrystals Guidance for Industry. US Dep Heal Hum Serv Food Drug Adm. 2016;(August):1–5.
- Food and Drug Administration. Regulatory classification of pharmaceutical cocrystals, guidance for industry. US Dep Heal Hum Serv. 2018;(February):1–4.
- Kumar A., Kumar S., Nanda A. A review about regulatory status and recent patents of pharmaceutical cocrystals. Adv. Pharm. Bull. 2018. 8(3): 355–63.
- Shemchuk O., Tsenkova B.K., Braga D., Duarte M.T., André V., Grepioni F. Ionic Cocrystal Formation as a Path Towards Chiral Resolution in the Solid State. Chem – A Eur J. 2018; 24(48): 12564–73.
- Duggirala N.K., Perry M.L., Almarsson Ö., Zaworotko M.J. Pharmaceutical cocrystals: Along the path to improved medicines. Chem Commun. 2016;52(4): 640–55.
- Long B., Verma V., Ryan K.M., Padrela L. Generation and physicochemical characterization of posaconazole cocrystals using Gas Antisolvent (GAS) and Supercritical Solvent (CSS) methods. J Supercrit Fluids. 2021; 170: 105134.
- Braga D., Maini L., Grepioni F. Mechanochemical preparation of cocrystals. Chem Soc Rev. 2013;42(18): 7638–48.
- Braga D., Grepioni F., Maini L., Prosperi S., Gobetto R., Chierotti M.R. From unexpected reactions to a new family of ionic cocrystals: The case of barbituric acid with alkali bromides and caesium iodide. Chem Commun. 2010;46(41): 7715–7.
- Yao J., Chen J.M., Xu Y.B., Lu T.B. Enhancing the solubility of 6-mercaptopurine by formation of ionic cocrystal with zinc trifluoromethanesulfonate: Single-crystal-to-single-crystal transformation. Cryst Growth Des. 2014;14(10): 5019–25.
- Kavanagh O.N., Croker D.M., Walker G.M., Zaworotko M.J. Pharmaceutical cocrystals: from serendipity to design to application. Drug Discov. Today. 2019;24(3). 796–804.
- Sun L., Zhu W., Yang F., Li B., Ren X., Zhang X., Hu W. Molecular cocrystals: Design, charge-transfer and optoelectronic functionality. Phys Chem Chem Phys. 2018;20(9): 6009-23.
- Hoogsteen K. The crystal and molecular structure of a hydrogen-bonded complex between 1-methylthymine and 9-methyladenine. Acta Crystallogr. 1963;16(9): 907–16.
- Buehler C.A., Heap A.G. A study of molecular organic compounds. I. The molecular organic compounds of meta-dinitrobenzene, 2,4-dinitrotoluene and 2,4-dinitrophenol. J Am Chem Soc. 1926;48(12):3168–72.
- Healy A.M., Worku Z.A., Kumar D., Madi A.M. Pharmaceutical solvates, hydrates and amorphous forms: A special emphasis on cocrystals. Adv. Drug Deliv. Rev.117. 2017: 25–46.
- Aitipamula S., Vangala V.R., Chow P.S., Tan R.B.H. Cocrystal hydrate of an antifungal drug, griseofulvin, with promising physicochemical properties. Cryst Growth Des. 2012;12(12): 5858–63.
- Kumar S., Nanda A. Approaches to Design of Pharmaceutical Cocrystals: A Review. Mol Cryst Liq Cryst. 2018; 667(1):54–77.
- Psimadas D., Georgoulias P., Valotassiou V., Loudos G. Molecular Nanomedicine Towards Cancer: J Pharm Sci. 2012;101(7): 2271–80.
- Basavoju S., Boström D., Velaga S.P. Indomethacin-saccharin cocrystal: Design, synthesis and preliminary pharmaceutical characterization. Pharm Res. 2008;25(3): 530–41.
- Zhang Y., Yang Z., Zhang S., Zhou X. Synthesis, crystal structure, and solubility analysis of a famotidine cocrystal. Crystals. 2019;9(7): 3–12.
- Childs S.L., Chyall L.J., Dunlap J.T., Smolenskaya V.N., Stahly B.C., Stahly G.P. Crystal engineering approach to forming cocrystals of amine hydrochlorides with organic acids. Molecular complexes of fluoxetine hydrochloride with benzoic, succinic, and fumaric acids. J Am Chem Soc. 2004;126(41):13335–42.
- Trask A. V., Samuel Motherwell W.D., Jones W. Pharmaceutical cocrystallization: Engineering a remedy for caffeine hydration. Cryst Growth Des. 2005;5(3): 1013–21.
- Bolla G., Nangia A. Pharmaceutical cocrystals: Walking the talk. Chem Commun. 2016;52(54): 8342–60.
- Mashhadi S.M.A, Yunus U., Bhatti M.H. Structural characterization and in-situ synthesis of quaternary ionic-cocrystal of isoniazid from un-ionized coformers. J Mol Struct. 2021;1233: 130015.
- Aitipamula S., Wong A.B.H., Chow P.S., Tan R.B.H. Novel solid forms of the anti-tuberculosis drug, Isoniazid: Ternary and polymorphic cocrystals. CrystEngComm. 2013;15(29): 5877–87.
- Aakeröy C.B., Salmon D.J. Building cocrystals with molecular sense and supramolecular sensibility. CrystEngComm. 2005;7: 439–48.
- Liu F., Song Y., Liu Y.N., Li Y.T., Wu Z.Y., Yan C.W. Drug-Bridge-Drug Ternary Cocrystallization Strategy for Antituberculosis Drugs Combination. Cryst Growth Des. 2018;18(3): 1283–6.
- Bhogala B.R., Nangia A. Ternary and quaternary cocrystals of 1,3-cis,5-cis- cyclohexanetricarboxylic acid and 4,4′-bipyridines. New J Chem. 2008;32(5): 800–7.
- Dunitz J.D., Bernstein J. Disappearing Polymorphs. Acc. Chem. Res. 1995; 28(4): 193-200.
- Aitipamula S., Chow P.S., Tan R.B.H. Polymorphism in cocrystals: A review and assessment of its significance. CrystEngComm. 2014;16(17): 3451–65.
- Mukherjee A., Desiraju G.R. Synthon polymorphism and pseudopolymorphism in cocrystals. The 4,4’-bipyridine–4-hydroxybenzoic acid structural landscape. Chem Commun. 2011;47(14): 4090–2.
- Braga D., Palladino G., Polito M., Rubini K., Grepioni F., Chierotti M.R., Roberto G. Three polymorphic forms of the cocrystal 4,4′-bipyridine/pimelic acid and their structural, thermal, and spectroscopic characterization. Chem – A Eur J. 2008;14(32): 10149–59.
- Li S., Chen J.M., Lu T.B. Synthon polymorphs of 1: 1 cocrystal of 5-fluorouracil and 4-hydroxybenzoic acid: Their relative stability and solvent polarity dependence of grinding outcomes. CrystEngComm. 2014;16(28): 6450–8.
- Jones W., Motherwell W.D.S., Trask A.V. Pharmaceutical cocrystals: An emerging approach to physical property enhancement. MRS Bull. 2006;31(11):875–9.
- Childs S.L., Hardcastle K.I. Cocrystals of piroxicam with carboxylic acids. Cryst Growth Des. 2007;7(7):1291–304.
- Kaupp G. Mechanochemistry: The varied applications of mechanical bond-breaking. CrystEngComm. 2009;11(3):388–403.
- Kumar A., Nanda A. In-silico methods of cocrystal screening: A review on tools for rational design of pharmaceutical cocrystals. J. Drug Deliv. Sci. 2021;63: 102527.
- Wood P.A., Feeder N., Furlow M., Galek P.T.A., Groom C.R., Pidcock E. Knowledge-based approaches to cocrystal design. CrystEngComm. 2014;16(26): 5839–48.
- Mohammad M.A., Alhalaweh A., Velaga S.P. Hansen solubility parameter as a tool to predict cocrystal formation. Int J Pharm. 2011;407(1–2): 63–71.
- Malamatari M., Ross S.A., Douroumis D., Velaga S.P. Experimental cocrystal screening and solution based scale-up cocrystallization methods. Adv. Drug Deliv. Rev. 2017;117: 162–77.
- Chan H.C.S., Kendrick J., Neumann M.A., Leusen F.J.J. Towards ab initio screening of cocrystal formation through lattice energy calculations and crystal structure prediction of nicotinamide, isonicotinamide, picolinamide and paracetamol multi-component crystals. CrystEngComm. 2013;15(19): 3799–807.
- Chadha, R., Saini, A., Arora, P. and Bhandari, S. Pharmaceutical cocrystals: a novel approach for oral bioavailability enhancement of drugs. Crit. Rev. Ther. Drug Carr. Syst. 2021;29(3)
- Allu S., Suresh K., Bolla G., Mannava M.K.C., Nangia A. Role of hydrogen bonding in cocrystals and coamorphous solids: indapamide as a case study. CrystEngComm. 2019;21(13): 2043–8.
- Lim, L.T., Auras, R. and Rubino, M. Processing technologies for poly (lactic acid). Prog. Polym. Sci. 2008; 33(8), 820-852.
- Salem A., Nagy S., Pál S., Széchenyi A. Reliability of the Hansen solubility parameters as cocrystal formation prediction tool. Int J Pharm. 2019;558: 319–27.
- Sarma B., Chen J., Hsi H.Y., Myerson A.S. Solid forms of pharmaceuticals: Polymorphs, salts and cocrystals. Korean J Chem Eng. 2011;28(2): 315–22.
- Etter M.C. Encoding and Decoding Hydrogen-Bond Patterns of Organic Compounds. Acc Chem Res. 1990;23(4): 120–6.
- Yuan G., Hui Z., Jianjun Z. Pharmaceutical cocrystals. Prog Chem. 2010;22(5): 829–36.
- Bis J.A., Zaworotko M.J. The 2-aminopyridinium-carboxylate supramolecular heterosynthon: A robust motif for generation of multiple-component crystals. Cryst Growth Des. 2005;5(3): 1169–79.
- Infantes L., Motherwell W.D.S. The probable number of hydrogen-bonded contacts for chemical groups in organic crystal structures. Chem Commun. 2004;4(10): 1166–7.
- Berry D.J., Steed J.W. Pharmaceutical cocrystals, salts and multicomponent systems; intermolecular interactions and property based design. Adv. Drug Deliv. Rev. 2017;117: 3–24.
- Roca-Paixão L., Correia N.T., Affouard F. Affinity prediction computations and mechanosynthesis of carbamazepine based cocrystals. CrystEngComm. 2019;21(45):6991–7001.
- Gajda M., Nartowski K.P., Pluta J., Karolewicz B. The role of the polymer matrix in solvent-free hot melt extrusion continuous process for mechanochemical synthesis of pharmaceutical cocrystal. Eur J Pharm Biopharm. 2018;131: 48–59.
- Gajda M., Nartowski K.P., Pluta J., Karolewicz B. Continuous, one-step synthesis of pharmaceutical cocrystals via hot melt extrusion from neat to matrix-assisted processing – State of the art. Int. J. Pharm. 2019; 558: 426–40.
- Ross S.A., Lamprou D.A., Douroumis D. Engineering and manufacturing of pharmaceutical cocrystals: A review of solvent-free manufacturing technologies. ChemComm. 2016;52(57): 8772–86.
- Kelly A.L., Gough T., Dhumal R.S., Halsey S.A., Paradkar A. Monitoring ibuprofen-nicotinamide cocrystal formation during solvent free continuous cocrystallization (SFCC) using near infrared spectroscopy as a PAT tool. Int J Pharm. 2012;426(1–2): 15–20.
- Rehder S., Christensen N.P.A., Rantanen J., Rades T., Leopold C.S. High-shear granulation as a manufacturing method for cocrystal granules. Eur J Pharm Biopharm. 2013;85(3 PART B): 1019–30.
- Alhalaweh A., Kaialy W., Buckton G., Gill H., Nokhodchi A., Velaga S.P. Theophylline cocrystals prepared by spray drying: Physicochemical properties and aerosolization performance. AAPS PharmSciTech. 2013;14(1): 265–76.
- Liu G., Li J., Deng S. Applications of supercritical anti-solvent process in preparation of solid multicomponent systems. Pharmaceutics. 2021;13(4): 475.
- Padrela L., Rodrigues M.A., Velaga S.P., Fernandes A.C., Matos H.A., de Azevedo EG. Screening for pharmaceutical cocrystals using the supercritical fluid enhanced atomization process. J Supercrit Fluids. 2010; 53(1–3): 156–64.
- Balbinot Filho C.A., Dias J.L., Rebelatto E.A., Oliveira J.V., Ferreira S.R.S., Lanza M. Precipitation of quercetin and nicotinamide in carbon dioxide + ethanol systems at high pressures: Phase equilibrium data for antisolvent processes. Fluid Phase Equilib. 2021;533: 112959.
- Wang I.C., Lee M.J., Sim S.J., Kim W.S., Chun N.H., Choi G.J. Anti-solvent cocrystallization of carbamazepine and saccharin. Int J Pharm. 2013;450(1–2): 311–22.
- Eddleston M.D., Patel B., Day G.M., Jones W. Cocrystallization by freeze-drying: Preparation of novel multicomponent crystal forms. Cryst Growth Des. 2013;13(10): 4599–606.
- Goyal S., Thorson M.R., Zhang G.G.Z., Gong Y., Kenis P.J.A. Microfluidic approach to cocrystal screening of pharmaceutical parent compounds. Cryst Growth Des. 2012;12(12): 6023–34.
- Apshingekar P.P., Aher S., Kelly A.L., Brown E.C., Paradkar A. Synthesis of Caffeine/Maleic Acid Cocrystal by Ultrasound-assisted Slurry Cocrystallization. J Pharm Sci. 2017;106(1): 66–70.
- Hossain Mithu M.S., Ross S.A., Hurt A.P., Douroumis D. Effect of mechanochemical grinding conditions on the formation of pharmaceutical cocrystals and co-amorphous solid forms of ketoconazole – Dicarboxylic acid. J Drug Deliv Sci Technol. 2021;63: 102508
- Ferreira P.O., de Almeida A.C., dos Santos É.C., Droppa R., Ferreira F.F., Kogawa AC, Caires F.J. A norfloxacin-nicotinic acid cocrystal: Mechanochemical synthesis, thermal and structural characterization and solubility assays. Thermochim Acta. 2020; 694: 178782.
- Yamamoto K., Tsutsumi S., Ikeda Y. Establishment of cocrystal cocktail grinding method for rational screening of pharmaceutical cocrystals. Int J Pharm. 2012;437(1–2): 162–71.
- Cao F., Amidon G.L., Rodriguez-Hornedo N., Amidon G.E. Mechanistic Analysis of Cocrystal Dissolution as a Function of pH and Micellar Solubilization. Mol Pharm. 2016;13(3): 1030–46.
- Kale D.P., Zode S.S., Bansal A.K. Challenges in Translational Development of Pharmaceutical Cocrystals. J Pharm Sci. 2017;106(2): 457–70.
- Kuminek G., Cavanagh K.L., Da Piedade M.F.M., Rodríguez-Hornedo N. Posaconazole Cocrystal with Superior Solubility and Dissolution Behavior. Cryst Growth Des. 2019;19(11): 6592–602.
- Biscaia I.F.B., Oliveira P.R., Gomes S.N., Bernardi L.S. Obtaining cocrystals by reaction crystallization method: Pharmaceutical applications. Pharmaceutics. 2021;13(6): 898.
- Li P., Chu Y., Wang L., Wenslow R.M., Yu K., Zhang H., Deng Z. Structure determination of the theophylline-nicotinamide cocrystal: A combined powder XRD, 1D solid-state NMR, and theoretical calculation study. CrystEngComm. 2014;16(15): 3141–7.
- Izutsu K.I., Koide T., Takata N., Ikeda Y., Ono M., Inoue M., Fukami T. Yonemochi E. Characterization and Quality Control of Pharmaceutical Cocrystals. Chem. Pharm. Bull. 2016;64(10): 1421-30.
- Qiao N., Li M., Schlindwein W., Malek N., Davies A., Trappitt G. Pharmaceutical cocrystals: An overview. Int. J. Pharm. 2011;41991-2): 1–11.
- Padrela L., De Azevedo E.G., Velaga S.P. Powder X-ray diffraction method for the quantification of cocrystals in the crystallization mixture. Drug Dev Ind Pharm. 2012;38(8): 923–9.
- Yousef M.A.E., Vangala V.R. Pharmaceutical cocrystals: molecules, crystals, formulations, medicines. Cryst Growth Des. 2019;19(12): 7420–38.
- Eddleston M.D., Thakuria R., Aldous B.J., Jones W. An investigation of the causes of cocrystal dissociation at high humidity. J Pharm Sci. 2014;103(9): 2859–64.
- Seo J.W., Hwang K.M., Lee S.H., Kim D.W., Park E.S. Preparation and characterization of adefovir dipivoxil–stearic acid cocrystal with enhanced physicochemical properties. Pharm Dev Technol. 2018;23(9): 890–9.
- Chavan R.B., Thipparaboina R., Yadav B., Shastri N.R. Continuous manufacturing of cocrystals: challenges and prospects. Drug Deliv. Transl. Res. 2018;8: 1726–39.
- Roy P., Ghosh A. Progress on cocrystallization of poorly soluble NME’s in the last decade. CrystEngComm. 2020;22(42): 6958–74.

This work is licensed under a Creative Commons Attribution 4.0 International License.





