How to Cite | Publication History | PlumX Article Matrix
Acceptance and Attitudes towards Covid-19 Vaccines: A Cross-Sectional Study from India
Sreedevi Sarsan 1* , Puppala Harshanya2
, Puppala Harshanya2 , Sunkara Anika2
, Sunkara Anika2 , Hesham Ali El Enshasy3, 4
, Hesham Ali El Enshasy3, 4  and R Z Sayyed5,6
and R Z Sayyed5,6
1Department of Microbiology, St. Pious X Degree and PG College for Women, Hyderabad, Telangana, India
2Department of MBA, St. Pious X PG (MBA) College for Women, Hyderabad, Telangana, India
3Institute of Bioproduct Development (IBD), Universiti Teknologi Malaysia (UTM), Skudai, Johor Bahru, Malaysia.
4City of Scientific Research and Technology Applications, New Burg Al-Arab, Alexandria, Egypt
5Department of Microbiology, PSGVP Mandal's, S I Patil Arts, G B Patel Science and STKV Sangh Commerce College, Shahada, India
6Faculty of Health and Life Sciences, INTI International University, Persiaran Perdana BBN, Putra Nilai, 71800 Nilai, Negeri Sembilan, Malaysia.
Corresponding Author E-mail:drsreedevi@stpiouscollege.org
DOI : http://dx.doi.org/10.13005/bbra/3172
ABSTRACT: COVID-19 is a pandemic caused by SARS-CoV-2 – 2 that caused a devastating impact and has affected human population globally. The mitigation measures to impede and contain the spread of the COVID-19 disease included lockdowns, social distancing, and use of masks, hand sanitization & other basic hygiene practices. Administration of vaccines was considered the most effective strategy to reduce the risk of the disease. Several vaccines developed against multiple variants of Coronavirus to combat the dreadful disease. The project was taken with the objective to know the perceptions of people about the COVID-19 vaccines and was intended to evaluate the awareness, attitude, and factors determining their acceptance or hesitancy towards the vaccine among the Indian population. This study was conducted using a snowball sampling technique employing a cross-sectional and web-based online survey. A self-administered questionnaire was prepared and circulated among the general Indian population and feedback was collected and analyzed on knowledge and awareness, attitudes, and perceptions about COVID-19 vaccines. A total of 1,507 respondents from different states of India have participated in the survey. A considerable percentage of the population approximately 90% was fully vaccinated due to concerted efforts of Government authorities and health officials. The majority of the people were hesitant and unsure to administer booster doses of vaccines. The results suggested that the majority of the Indian population had good knowledge and awareness about Covid 19 vaccines and had a very positive attitude and acceptance towards vaccines but showed a lack of interest towards booster doses. Hence, various strategies need to be formulated to update the information to the public through awareness programs and their effective implementation.
KEYWORDS: Acceptance; Attitudes; Booster Doses COVID-19; Coronavirus; Hesitancy; SARS-CoV – 2; Vaccines; India
Download this article as:| Copy the following to cite this article: Sarsan S, Harshanya P, Anika S, El-Enshasy H. A, Sayyed R. Z. Acceptance and Attitudes towards Covid-19 Vaccines: A Cross-Sectional Study from India. Biotech Res Asia 2023;20(4). |
| Copy the following to cite this URL: Sarsan S, Harshanya P, Anika S, El-Enshasy H. A, Sayyed R. Z. Acceptance and Attitudes towards Covid-19 Vaccines: A Cross-Sectional Study from India. Biotech Res Asia 2023;20(4). Available from: https://bit.ly/4ajPCeR |
Introduction
COVID-19 is a pandemic caused by SARS-CoV – 2 (Severe Acute Respiratory Syndrome Corona Virus 2). The virus first emerged in late 2019 in Wuhan (Hubei, China) and has swiftly spread to every continent and has affected the human population globally.1,2 Corona virus transmits mainly through droplet mode of transmission and once internal to a human body it affects the respiratory system & in severe cases it is fatal. The pandemic has potentially impacted education, business, politics, the environment, and all other sectors of human life. COVID-19 is much more than a health crisis and has potentially created devastating social, economic, and political crises that had a large impact on human life. The devastating impact caused by the pandemic urged the necessity to adopt and practice a few mitigating policies to contain the adverse effects of the pandemic.3,4 The mitigation measures to prevent widespread transmission of the coronavirus included lockdowns (partial/complete), social distancing, use of masks, hand sanitization, and other basic hygiene practices.4,5 These measures have become mandatory to contain the transmission and are challenging for all the government regulatory bodies to implement the measures. Although these measures could reduce the disease progression and fatality rates, the best strategy to limit the pandemic was to develop antiviral agents and vaccines that were safe and effective apart from being affordable. Thus, vaccines are the safest and most reliable, and effective intervention that can potentially treat COVID-19 and reduce the high burden of disease globally.6,7 There are multiple variants of Corona virus and several vaccines have been developed as of December 2020 and declared successful and approved for use to combat the dreadful disease COVID-19.4,8 Ten Vaccines were approved for use by WHO as per the information received on Jan 2022 – RNA based (Spikevax, Comirnaty); Protein Subunit (Nuvaxovid, Covovax), Non Replicating Viral Vector based (Ad26.COV2.S , Vaxzevria, Covishield); Inactivated vaccines (Covaxin, Covilo, CoronaVac).
As per statistics quoted in Business Standard newspaper dated 31st December, 2021, India’s 90% of the adult population was vaccinated against COVID-19.9 India began its vaccination program on 16 January 2021, primarily with two vaccines namely Covaxin and Covishield manufactured by Bharat Biotech and Serum Institute of India respectively. The vaccines were developed under the Public Private Partnership (PPP) model within a span of one year with a clinical efficacy of around 81% for Covaxin and 63.09% for Covishield.10,11 In the initial stages, one of the two vaccines, approved for restricted emergency use, was administered to both healthcare industry professionals and workers along with medical students. Eventually, from March 1, 2021, onwards, COVID-19 vaccines were provided to people >60 years of age and also to those with comorbidities in the age group of 45-59 years. An online portal CO-WIN (COVID-19 Vaccine Intelligence Network) was developed with the support of the United Nations Development Program (UNDP) and made accessible to the public for the registration process for vaccination. The COWIN portal was configured to track the user data of the vaccinated beneficiaries, provide reminders via SMS, and also to issue vaccination certificates. However, despite the unprecedented speed of vaccine development and robust mass vaccination efforts, vaccine acceptance and uptake have been suboptimal in certain parts of the country, especially among uneducated and rural population.12
Vaccine hesitancy is defined as the reluctance or denial to receive vaccines in spite of vaccine availability and accessibility. This may ultimately hamper the vaccination process and become a major threat in tackling the diseases preventable by vaccination.13 The terms vaccine hesitancy and vaccine resistance are used when the user is unsure and objects to get vaccinated respectively. The majority of the unvaccinated population comprises vaccine hesitancy rather than vaccine resistance individuals. Vaccine hesitancy is a multi-faceted and context-dependent phenomenon and varies according to disease, time, and place. This vaccine hesitancy can be tackled by means of certain characteristics such as confidence, complacency, convenience, communication, and context.14 Vaccine hesitancy is considered the most important hurdle to overcome towards attaining the threshold for herd immunity.15 Because the options available for COVID-19 treatment were not very effective, public health experts firmly suggested that mass vaccination would be the most viable strategy in order to combat the COVID-19 pandemic.16 Despite the effective and safe vaccines that were developed, there was mounting skepticism about the whole process of vaccination.
Therefore, it is crucial, and urgent need to understand and comprehend the attitudes of people towards vaccines. It is also imperative to identify the various factors that impact the rationale of people’s decisions towards vaccines, so as to appropriately modify the strategies for the benefit of public health. Thus, this study was conducted with the aim to examine the various factors that impact vaccine hesitancy and vaccine acceptance and also to investigate the association between the various socio-demographic characteristics, health status, attitudes, and awareness/ knowledge regarding COVID-19 vaccines among the Indian population.
Methodology
Study Design and Sample Size
The study was based on a cross-sectional survey and was conducted in October 2021. The study employed a convenient sample approach which included participants from Telangana as well as other states of India. The questionnaire included 40 questions and was framed with the purpose of collecting information about basic demographic details, awareness/ knowledge, information sources, attitudes and perception towards COVID-19 vaccines, perceived symptoms after vaccination and prior vaccination experience, and acceptance of booster dose. Based on the previously available literature on vaccine acceptance, we arrived at a sample size of 1500 with a confidence limit of 95% using the formula N = Zα2P (1 − P)/d2 where values of α, Zα, P, and d were 0.05, 1.96, 50%, and 0.1. A self-administered online questionnaire was prepared in Google Forms and circulated using the snowball sampling method on various social media platforms (WhatsApp, Facebook, and e-mail).
Study Area and Participants
The study was an online questionnaire survey conducted among the population belonging to different states in India, especially Telangana. A questionnaire for an online survey was developed based on a literature review and was communicated to various people belonging to the age group of 18 to 60 using social media and other applications such as WhatsApp, Telegram, and E-mail.
Questionnaire Development
A self-administered questionnaire was developed based on literature review and peer discussion. The questionnaire was developed in English language and contained multiple-choice questions that required approximately 15-20 minutes to complete. A presurvey check was done to validate the questionnaire and thus was tested among 30 random individuals (pilot sample n=30) and was improvised and edited for any further inclusion and exclusion. The final questionnaire consisted of a total of 40 questions and was structured into 5 sections (Supplementary File 1). The first part of the questionnaire comprised 12 questions on personal information such as name, age, gender, marital status etc. The second part of the questionnaire dealt with personal habits like smoking status, current illness if any and type of treatment or medication for the same. The third part of the questionnaire were on the knowledge on COVID-19, usage of Aarogya Setu app, previous infection with COVID-19 among members of the family, etc. The fourth part of the questionnaire dealt with information on the type of treatment undertaken and precautions that were taken during the pandemic. The last part of the questionnaire comprised questions based on attitude, acceptance, and knowledge of COVID-19 vaccines, such as reasons for not taking vaccines, confidence in the vaccine, and type of illness that followed after vaccination, etc. It was mandated to answer all the questions in the survey form in order to avoid incomplete or missing data.
Collection of Data
The final questionnaire was deployed online using Google Forms and was circulated to participants employing the snowball sampling method using various social media platforms like WhatsApp, Facebook, and e-mail. Participants were even requested to pass on the survey form to their acquaintances or contact people. The questionnaire was communicated to people above 18 years of age from various regions of Telangana as well as other states of India. It was a completely voluntary participation and were not offered any kind of reward. The data was collected for a time period of 3 months between 1 October, 2021 to 31 December 2021. A total of 1507 responses were gathered. Once the desired sample size was achieved, no new entries were made. The data collected from the survey was recorded in MS Excel and incomplete responses were removed to get proper data for analysis.
Data Analysis
The raw data which was collected using the survey form were coded and entered into specially designed databases (Microsoft Access). The coded data was then transported to Microsoft Excel spreadsheets and statistically analyzed using SPSS software. The descriptive variables were represented as mean (standard deviation), frequency, and percentages.
Ethical Considerations
The participants were informed regarding the purpose of the survey through a statement added at the beginning of the online form. The confidentiality and privacy of the data were maintained throughout the process of the study. The personal information of the respondents was collected and stored in secured folders that were accessible only by the researchers and the data was protected from the unauthorized personnel.
Results
A total of 1,507 subjects were involved in the study belonging to different states of India and 85% of the responses were from Telangana state. Detailed demographics are presented in Table 1. The mean age of the subjects was found to be 27±9.5 years. The majority of the respondents were between 20 and 30 years of age. Out of the 1,507 participants, more than 80% of the participants were female. With respect to educational status, 55% of the respondents were graduates while 32% of them were post-graduates. The rest of the respondents reported to have completed their secondary schooling. Out of all the participants, 26% of them were unemployed while the rest of them were employed as teachers, healthcare professionals, software professionals, etc. Further, 75% of the respondents were unmarried. With respect to the size of the family, the majority of the participants (n=730) declared that their family consisted of four members.
Table 1: Demographic details of study participants (n=1507)
| VARIABLE | DATA | |
| Age in Years | No. of Respondents | % |
| Below 20 | 549 | 36.40% |
| 20-30 | 616 | 40.80% |
| 30-40 | 137 | 9.09% |
| 40-50 | 125 | 8.29% |
| 50-60 | 44 | 2.91% |
| 60-70 | 25 | 1.65% |
| 70 and above | 11 | 0.72% |
| Gender | ||
| Male | 263 | 17.45% |
| Female | 1244 | 82.54% |
| Transgender | 0 | 0 |
| Marital Status | ||
| Married | 371 | 24.60% |
| Unmarried | 1136 | 75.40% |
| Educational Background | ||
| Primary | 1 | 0.07% |
| Secondary | 171 | 11.34% |
| Graduate | 843 | 55.90% |
| Postgraduate and above | 488 | 32.30% |
| Illiterate | 4 | 0.26% |
| Occupation | ||
| Unemployed | 405 | 26.8 |
| Healthcare | 55 | 3.64% |
| Police Personnel | 0 | 0 |
| Software Professionals | 60 | 3.98% |
| Sanitation Workers | 0 | 0 |
| Banking | 0 | 0 |
| Teaching | 163 | 10.81% |
| Self Employed (Business) | 67 | 4.44% |
| Others | 757 | 50.23% |
| Smoking status | ||
| Smoker | 18 | 1.19% |
| Occasional Smoker | 25 | 1.65% |
| Ex-smoker | 24 | 1.59 |
| Non smoker | 1440 | 95.60% |
| Place of Residence | ||
| Village | 1118 | 74.10% |
| Town | 227 | 15.06% |
| City | 162 | 10.70% |
| Use of Aarogya Setu app | ||
| Yes | 562 | 37.29% |
| No | 945 | 62.70% |
| Any Illness | ||
| No illness | 1324 | 87.80% |
| Suffering from illness | 183 | 12.14% |
|
People tested positive for COVID-19 |
||
| Nil | 1176 | 78.03% |
| Once | 292 | 19.37% |
| Twice | 39 | 2.58% |
In the current study, more than 95% of the participants were non-smokers. Only 37% of the respondents reported to have been using Aarogya Setu mobile app and 87% of them reported have been not diagnosed with any illness. Among the participants who were suffering from other illnesses, blood pressure, thyroid issues, and diabetes were predominant followed by PCOD, heart diseases, liver diseases, and kidney disorders. Further, 86% of the respondents also reported that they were not under any treatment or medications. As regards the precautionary measures followed during the pandemic, 12% of the respondents reported that had followed none of the precautions such as wearing masks, washing hands, sanitizer, etc. Further, 84% of the respondents reported having used hand sanitizer and face masks, and 14% reported having used Ayurvedic medicines as a precaution against COVID-19 (Fig 1).
 |
Figure 1: Graphical Representation of Choice of Precautionary Measures for COVID. |
In this study, 23% of the respondents reported having been consuming some medicines to improve immunity. Among the 1,507 participants, only 19% reported having been diagnosed with COVID-19 once, and less than 3% reported having been diagnosed with COVID-19 respectively. Among the respondents who were previously diagnosed with COVID-19, the major symptoms observed as self-reported by the participants were cold (9%), cough (9%), fever (12.7%), body pain (10.5%), headache (9%) and sore throat (6%). Among the 331 respondents previously diagnosed with COVID-19, around 55% of the infected were during the second wave of COVID-19 in India i.e., between February 2021 to July 2021. The place of treatment was home for 84% of the participants who were previously diagnosed with COVID-19 while the rest of them were treated in either government or private hospitals (Fig 2 & 3). None of them reported to have been infected with COVID-19 during pregnancy. Data collected on COVID-19 infections among the family members of the respondents revealed that around 52% of the respondents’ family members were infected during the pandemic and more than 90% of them reported having at least two members of the family infected with COVID-19. Around 40% of the infections among family members were in the age group of 40-60 years (Fig 4 & 5).
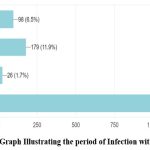 |
Figure 2: Bar Graph Illustrating the period of Infection with COVID 19 |
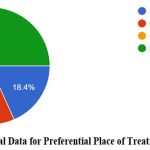 |
Figure 3: Graphical Data for Preferential Place of Treatment for COVID 19. |
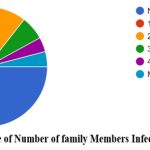 |
Figure 4: Percentage of Number of family Members Infected with COVID 19. |
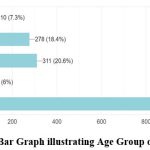 |
Figure 5: Bar Graph illustrating Age Group of Infection. |
Out of the 1,507 participants, around 89% of the participants were fully vaccinated with two doses. The association coefficient between education level and vaccination intake was done using a chi-square test and was found to be statistically significant at a 5% level of significance (Table 2). Among them, only 66% of the respondents were confident that the vaccine provided the necessary immunity against COVID-19. 75% of the respondents have been administered with Covishield vaccine and 21% have been vaccinated with Covaxin and a meager percentage have administered other types of vaccines (Fig 6 & 7). Around 91% of the vaccinated respondents reported that they had taken the first and second doses of the same vaccine while the remaining reported to have taken mixed doses. Only 10 respondents reported having taken the Sputnik vaccine. Around 70% of the vaccinated respondents reported having taken their vaccine from a government health center, while the remaining had been vaccinated from private hospitals. The source of information for the COVID-19 vaccine was news (58%), social media (26%) and family and friends (16%) respectively. Among the 102 respondents who did not take the vaccine, 32 respondents replied that “personal choice” was a reason for not taking the vaccine, followed by “waiting for other vaccines to be developed” (n=15) and “distrust in the vaccine as they were developed quickly” (n=10).
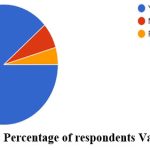 |
Figure 6: Percentage of respondents Vaccinated. |
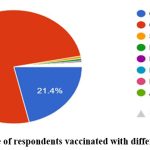 |
Figure 7: Percentage of respondents vaccinated with different types of vaccines |
More than 50% of the vaccinated participants reported that they felt sick after receiving the first dose while 16% reported to have felt sick both the times after vaccination. Predominant post-vaccination side effects observed were pain or swelling in the injected site, fever, irritability, headache, and body pain. With respect to break-out infections, among the 1320 respondents vaccinated against COVID-19, 57 reported having been infected with COVID-19 after the first dose while 33 had reported to had an infection after the second dose. The association between the type of vaccine and break-out infection after getting at least one dose of either vaccine was done using a chi-square test and was found to be statistically significant (Table 3). All the respondents have reported that they had taken their two doses of vaccine after the prescribed time interval from the Government of India i.e., 28 days for Covaxin and 84 days for Covishield. More than 95% of the respondents reported that they continued to follow safety precautions like social distancing, and usage of face masks and hand sanitizer even after being vaccinated. Responses for the acceptability of booster dose revealed that around 45% of the respondents replied “maybe” while only 30% were confident of getting the booster dose whereas the remaining (25%) replied “no”. The association between the education level and acceptance to take booster doses was done using a chi-square test and was found to be statistically significant (Table 4).
Table 2: Association between education level and vaccination intake
| Education level | Vaccinated for COVID-19 | ||
| Yes | No | Planning to take | |
| Illiterate | 4 (100%) | 0 (0.0%) | 0 (0.0%) |
| Primary | 1 (100%) | 0 (0.0%) | 0 (0.0%) |
| Secondary | 129 (75.4%) | 29 (16.7%) | 13 (7.6%) |
| Graduate | 737 (87.4%) | 58 (6.9%) | 48 (5.7%) |
| Postgraduate & above | 475 (97.3%) | 4 (0.8%) | 9 (1.8%) |
Trend Chi-square p-value=<0.001
Table 3: Association between type of vaccine and break out infection
| Type of vaccine | Breakout infection | |
| Yes | No | |
| Covishield | 61 (6.1%) | 947 (93.9%) |
| Covaxin | 17 (6.0%) | 266 (94.0%) |
Chi-square p-value= 0.978
Table 4: Association between education level and booster acceptance
| Education level | Prefer taking booster dose of vaccine | ||
| Yes | May be | No | |
| School or below | 36 (26.1%) | 67 (48.6%) | 35 (25.4%) |
| Graduate & above | 373 (30.3%) | 548 (44.5%) | 311 (25.2%) |
Trend Chi-square p-value=0.545
Discussion
The government has urged all Indian citizens to be immunized against COVID-19 disease, the largest vaccination program to be launched in the world. The free vaccination program was initiated in India on 16 January 2021. In this background, our study aimed to assess the vaccine confidence, hesitancy, and attitude towards vaccination among the Indian population, particularly of Telangana State. Since the questionnaire was in English, it was circulated only among the educated group of participants. The results from the present study showed that more women participated in the study compared to men. A study conducted in UK adults found that smokers showed unwillingness and were more hesitant towards Covid-19 vaccines.17 No such observations were made in our study since a majority of the respondents were non-smokers. Recent studies also revealed lowered immune response among vaccinated adults against COVID-19 due to smoking.18, 19
The usage of the Aarogya Setu mobile phone application was reported to be high among the residents of Delhi and Kerala when compared to other states of India.20, 21 In a previous study conducted, educational levels had an impact on the awareness related to Aarogya Setu app.22 However, no such association was found in the present study where the majority of the respondents had higher levels of education. In the present study, an association was found between educational level and vaccine intake. Studies conducted elsewhere showed that individuals with no education or with a basic secondary education were probably the least interested in receiving the COVID-19 vaccine when compared to Graduates.23 Similar observations were made in earlier studies related to the association between education and the acceptability of COVID-19 vaccines.24 In this study, more than 85% of the respondents who have taken the vaccine reported having a graduate degree. However, no such association was found between educational levels and booster dose acceptance among the participants since a majority of them were not sure about taking the booster dose. As of May 9, 2022, only about 2.1 percent of the total population of India has gotten a booster dose.25 In contrast, a study assessing the booster dose acceptability conducted in UAE reported that highly educated individuals showed a more positive inclination towards COVID-19 vaccination and a greater willingness to take booster doses.26
Earlier studies had shown that common side effects after vaccinating with Covaxin and Covishield were injection site pain, tenderness, fever, headache, fatigue, and myalgia.27, 28 similar side effects were reported by respondents in the present study as well. However, the drugs used for chemoprophylaxis such as ivermectin and vaccine-related serologic responses were not taken into consideration during the survey.29,30,31 The infection, in which a person becomes infected again when exposed to the same pathogen, in spite of fully vaccinated, is called as breakthrough infection. With respect to breakout infection, only 6% of the participants who had received both doses of either of the vaccine reported to have been again diagnosed with COVID-19.
Conclusion
In conclusion, India is one of the preeminent countries with respect to acceptance of individuals towards COVID-19 vaccines. Despite the unprecedented speed of vaccine development and robust mass vaccination efforts, vaccine acceptance and uptake have been suboptimal in certain parts of the country, especially among the uneducated and rural population. However, in India, a considerable percentage of the population (approximately 90%) was fully vaccinated due to concerted efforts of Government authorities and health officials. The chief reason for the acceptance of vaccines among the population was the availability of vaccines free of cost or subsidized rates provided by the government. The majority of the people were hesitant and unsure to administer booster doses of vaccines. Hence, the government authorities should intervene and design social media campaigns to create more awareness, in general, and also for specific population segments, about the safety and efficacy of the vaccines and booster doses not only for COVID-19 and its emerging variants but also for other microbial diseases. In the future, awareness should be created among the public with a focus on the technology used in the production of vaccines so as to increase the level of acceptance towards vaccines and booster doses.
Acknowledgement
We thank the Management and Principal Dr. Sr. B. Velangini of St. Pious X Degree and PG College for Women, Hyderabad for giving us permission to carry out this project and supporting us to complete the project.
Conflict of Interest
There is no conflict of interest .
Funding Sources
There is no funding sources
References
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard: World Health Organization; 2020.
- Helmy YA, Fawzy M, Elaswad A, Sobieh A, Kenney SP, Shehata AA. The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. Journal of clinical medicine. 2020 Apr 24;9(4):1225.
CrossRef - Phua J, Weng L, Ling L, Egi M, Lim CM, Divatia JV, et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. The lancet respiratory medicine. 2020;8(5):506-17.
CrossRef - El-Elimat T, AbuAlSamen MM, Almomani BA, Al-Sawalha NA, Alali FQ. Acceptance and attitudes toward COVID-19 vaccines: A cross-sectional study from Jordan. Plos one. 2021;16(4):e0250555.
CrossRef - Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, et al. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. International journal of surgery. 2020;78:185-93.
CrossRef - Ehreth J. The value of vaccination: a global perspective. Vaccine. 2003;21(27):4105-17.
CrossRef - Rodrigues CM, Plotkin SA. Impact of vaccines; health, economic and social perspectives. Frontiers in microbiology. 2020;11:1526.
CrossRef - Faezi NA, Gholizadeh P, Sanogo M, Oumarou A, Mohamed MN, Cissoko Y, Sow MS, Keita BS, Baye YA, Pagliano P, Akouda P. Peoples’ attitude toward COVID-19 vaccine, acceptance, and social trust among African and Middle East countries. Health Promotion Perspectives. 2021;11(2):171.
CrossRef - https://www.business-standard.com/article/current-affairs/90-of-adult-population-in-india-vaccinated-with-first-dose-govt-121123000915_1.html
- Thiagarajan K. What do we know about India’s Covaxin vaccine?. BMJ: British Medical Journal (Online). 2021:20:373.
CrossRef - Gómez CE, Perdiguero B, Esteban M. Emerging SARS-CoV-2 variants and impact in global vaccination programs against SARS-CoV-2/COVID-19. Vaccines. 2021;9(3):243.
CrossRef - Umakanthan S, Patil S, Subramaniam N, Sharma R. COVID-19 vaccine hesitancy and resistance in India explored through a population-based longitudinal survey. Vaccines. 2021;9(10):1064.
CrossRef - MacDonald NE. Vaccine hesitancy: Definition, scope and determinants. Vaccine. 2015;33(34):4161-4.
CrossRef - Razai MS, Oakeshott P, Esmail A, Wiysonge CS, Viswanath K, Mills MC. COVID-19 vaccine hesitancy: the five Cs to tackle behavioural and sociodemographic factors. Journal of the Royal Society of Medicine. 2021;114(6):295-8.
CrossRef - Gerretsen P, Kim J, Caravaggio F, Quilty L, Sanches M, Wells S, et al. Individual determinants of COVID-19 vaccine hesitancy. PLoS One. 2021;16(11):e0258462.
CrossRef - Fadda M, Albanese E, Suggs LS. When a COVID-19 vaccine is ready, will we all be ready for it?. International journal of public health. 2020;65(6):711-2.
CrossRef - Jackson SE, Paul E, Brown J, Steptoe A, Fancourt D. Negative vaccine attitudes and intentions to vaccinate against Covid-19 in relation to smoking status: A population survey of UK adults. Nicotine and Tobacco Research. 2021;23(9):1623-8.
CrossRef - Ferrara P, Gianfredi V, Tomaselli V, Polosa R. The effect of smoking on humoral response to COVID-19 vaccines: a systematic review of epidemiological studies. Vaccines. 2022;10(2):303.
CrossRef - Mori Y, Tanaka M, Kozai H, Hotta K, Aoyama Y, Shigeno Y, Aoike M, Kawamura H, Tsurudome M, Ito M. Antibody response of smokers to the COVID-19 vaccination: Evaluation based on cigarette dependence. Drug Discoveries & Therapeutics. 2022;16(2):78-84.
CrossRef - Rout K, Sahu S. Exploring factors influencing the users’ intention to use Aarogya Setu contact tracing mobile health application during COVID-19 pandemic. Horizon. 2020;2:29-36.
CrossRef - Jose R, Benny PV, Narendran M, Bindu A. Perceptions towards COVID 19 and Reflections on Aarogya Setu Mobile Application Use among Keralites. Kerala Medical Journal. 2020;13(2):57-61.
- Gupta P, Gupta A, Dixit S, Kumar H. Knowledge, attitude, and practices regarding COVID-19: A cross-sectional study among rural population in a northern Indian District. Journal of Family Medicine and Primary Care. 2020;9(9):4769.
CrossRef - Soares P, Rocha JV, Moniz M, Gama A, Laires PA, Pedro AR, et al. Factors associated with COVID-19 vaccine hesitancy. Vaccines. 2021;9(3):300.
CrossRef - Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, Rabin K, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nature medicine. 2021;27(2):225-8.
CrossRef - https://www.thehindubusinessline.com/data-stories/data-focus/at-2-covid-booster-dose-coverage-is-among-the-lowest-in-india/article65397218.ece, May 2022.
- Jairoun AA, Al-Hemyari SS, El-Dahiyat F, Jairoun M, Shahwan M, Al Ani M, et al. Assessing public knowledge, attitudes and determinants of third COVID-19 vaccine booster dose acceptance: current scenario and future perspectives. Journal of Pharmaceutical Policy and Practice. 2022;15(1):1-3.
CrossRef - Parida SP, Sahu DP, Singh AK, Alekhya G, Subba SH, Mishra A, et al. Adverse events following immunization of COVID‐19 (Covaxin) vaccine at a tertiary care center of India. Journal of medical virology. 2022;94(6):2453-9.
CrossRef - Pokharel K, Dawadi BR, Karki A. Side Effects after Second Dose of Covishield Vaccine among Healthcare Workers: A Descriptive Cross-sectional Study. JNMA: Journal of the Nepal Medical Association. 2021:59(238):577.
CrossRef - Shouman, W. M., Hegazy, A. A., Nafae, R. M., Ragab, M. I., Samra, S. R., & Ibrahim, D. A. Use of ivermectin as a potential chemoprophylaxis for COVID-19 in Egypt: a randomized clinical trial. J Clin Diagn Res. 2021:15(2):10-7860.
CrossRef - Hegazy, A. A. Ivermectin for COVID-19 prophylaxis. European Journal of Clinical and Experimental Medicine. 2021:19 (3):280–282
CrossRef - Gerhards C, Thiaucourt M, Hetjens M, Haselmann V, Neumaier M, Kittel M. Heterologous Vector—mRNA Based SARS-CoV-2 Vaccination Strategy Appears Superior to a Homologous Vector—Based Vaccination Scheme in German Healthcare Workers Regarding Humoral SARS-CoV-2 Response Indicating a High Boosting Effect by mRNA Vaccines. Vaccines. 2023; 11(3):701.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





