Manuscript accepted on : 25-12-2023
Published online on: 12-01-2024
Plagiarism Check: Yes
Reviewed by: Dr. Kruti Dave
Second Review by: Dr. Rabindra Kumar
Final Approval by: Dr. Fabián Fernández-Luqueño
Briska Jifrina Premnath , Manoj Kumar Srinivasan
, Manoj Kumar Srinivasan , and Namasivayam Nalini*
, and Namasivayam Nalini*
Department of Biochemistry and Biotechnology, Faculty of Science, Annamalai University, Tamil Nadu, India
corresponding Author E-mail: nalininam@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/3217
ABSTRACT: Reduced graphene oxide (rGO) is a carbon nanomaterial with unique characteristics that permit application in numerous fields. Rutin is a flavonoid with a variety of biological functions and pharmaceutical applications. In topical years, a handful of research has stated on the environmental impacts of carbon nanoparticles (NPs) and the consequences of reduced graphene oxide on the species that live in water bodies. However, the capacity to recuperate after exposure is still mostly unfamiliar. This study analyzed the protective effect of rutin against rGO NPs in zebrafish and the optimal dose required to inverse the impairment caused by rGO NPs exposure in zebrafish. In this study, fish were treated for 14 days and 8 study groups were examined: control, rGO exposure alone (10 mg/L), rutin exposure alone (50, 100 and 200 mg/L) and rGO combined with 3 distinct rutin doses (10 mg/L of rGO + rutin 50, 100, and 200 mg/L). In the zebrafish gill tissues, rGO impairs cells by increasing LPO levels and inducing oxidative stress by suppressing antioxidants (SOD, CAT, GPx, GSH, GR, GST, and vitamin C). Multiple alterations, including sinusoidal and venous congestion, vacuole formation or cytoplasmic vacuolation of hepatocytes, an enlarged hepatic plate gap, and necrosis, were revealed on the structural examination of liver tissues. Based on our results, we conclude that sub-lethal doses of rGO (10 mg/L) could be harmful to zebrafish. Rutin supplementation between 100 and 200 mg/L can protect against the toxic effects of rGO, even though rGO is detrimental to the exposed fish population.
KEYWORDS: Antioxidants; Behaviour; Histology; Nanoparticle; rGO; Rutin; Toxicity; Zebrafish
Download this article as:| Copy the following to cite this article: Premnath B. J, Srinivasan M. K, Nalini N. Effect of Rutin on the Behavioural, Biochemical and Liver Morphological Changes in Danio Rerio Exposed to Reduced Graphene Oxide (Rgo) Nanoparticles. Biotech Res Asia 2024;21(1). |
| Copy the following to cite this URL: Premnath B. J, Srinivasan M. K, Nalini N. Effect of Rutin on the Behavioural, Biochemical and Liver Morphological Changes in Danio Rerio Exposed to Reduced Graphene Oxide (Rgo) Nanoparticles. Biotech Res Asia 2024;21(1). Available from: https://bit.ly/4atnPYH |
Introduction
Graphene family materials (GFM) are presently regarded as one of the most fascinating nanomaterials with numerous applications. Graphene is the fundamental structural component of a variety of carbon allotropes. Graphite (GR) is composed of graphene sheets arranged in sequential stacks through carbon-carbon bonds with sp2-hybridization. The arrangement of carbon atoms in graphene forms a honeycomb lattice, with van der Waals interactions binding adjacent planes within each layer. Consequently, the exfoliation of GR yields isolated graphene sheets, which can be accomplished by chemical and mechanical methods 1.
Unlike graphene, graphene oxide (GO) has functional groups, including carboxylic acid, epoxide, and hydroxyl, attached to a carbon sheet 2. The reduction can partially remove these functional groups 3. Thermal, chemical, or UV treatment of GO produces reduced graphene oxide (rGO) with fewer O2 groups and enhanced electrical conductivity 4. After reduction, rGO resembles an isolated graphene sheet; the existence of O groups in its structure only makes the difference 5, 6.
Graphene has become a robust material for the twenty-first century owing to its extraordinary characteristics, such as notable mechanical strength, impressive flexibility, highly efficient electrical/thermal conductivity, and chemical stability7. As drug carrier materials and scaffolds, graphene and its byproducts have been extensively evaluated in various applications, particularly biomedicine. In addition, it is employed in gene delivery, biosensors, tissue engineering, and neurosurgery8.
Rutin (3, 3′, 4′, 5, 7- pentahydroxyflavone-3-rhamnoglucoside) is a bioflavonoid present in various natural sources, like fruits and vegetables, especially buckwheat, tea, apple and passion flower9. It has numerous biological functions and pharmaceutical applications, including antioxidant, antiproliferative, anti-inflammatory, antiviral, and anticarcinogenic properties. While inhibiting platelet aggregation, it reduces hyperlipidemia. In addition, its low toxicity makes it an excellent candidate for potential clinical applications 10-12.
Owing to its exceptional structural and biological characteristics, zebrafish (Danio rerio) has been employed as an in vivo model to investigate the environmental health and safety (EHS) implications of engineered nanomaterials and nano-related products 13. Thereafter, many remarkable research using embryos, larvae, adults, and transgenic zebrafish have been carried out on accumulation, morphology, behaviour, neurological disorders, molecular mechanisms, and other potential targets of GPN 14-19.
In recent years, a few studies have explored carbon nanoparticles’ environmental implications and investigated GO and rGO’s toxicity across various cell types (bacterial, mammalian, and plant) and animal models (mice and zebrafish). Moreover, these studies have collected data on the impact of GFNs in both soil and aquatic ecosystems 20-23. The specific mechanism by which graphene derivatives interact with cells remains unclear; however, for GO, its toxicity is impacted by factors like the amount and type of O2-containing functional groups and the size, charge, and aggregation of the sheets2. Zhu et al.24 found that the aggregation of Buckminster-fullerene (C60) decreased the survival and hatching rates of zebrafish. Additionally, Zhang et al.25 and Yan et al.26 examined the effects of graphene and rGO on human health. Carbon nanoparticles have an impact on human health, plants, and animals. However, the specific toxicity of rGO in the water ecosystem remains unknown.
The objective of this research was to identify the optimum dose of rutin for mitigating the harm resulted by exposure to rGO NPs in zebrafish, a well-established vertebrate model for toxicity evaluation, by assessing behavioural responses, biochemical parameters, and morphological alterations.
Methodology
Synthesis and Characterization of rGO NPs
GO was formed using Hummer’s technique 27. Initially, 1g of natural graphite powder and 1g of NaNO3 were combined with a specific quantity of H2SO4 at 15°C, resulting in a suspension. Subsequently, 6g of KMnO4, a potent oxidizing agent, was gradually added to the suspension, and the mixture was continuously stirred for 2 hours in an ice bath while maintaining the temperature below 20°C. The mixture was stirred until it exhibited a brownish tint. After KMnO4 had completely dissolved, the temperature was maintained at 35°C for an additional 30 minutes. The suspension was then diluted with deionized water, and a specific amount of 30% H2O2 was introduced with continuous stirring, resulting in a bright yellow-coloured suspension. To eradicate any remaining unreacted salt, the mixture underwent treatment with 10% HCl and was rinsed multiple times with distilled water. After centrifugation, a gel-like solution was obtained and subsequently dried for 24 hours at 70°C in a hot air oven, yielding GO powder. rGO was produced as a brownish-black powder through thermal reduction at 200°C for three hours. The produced reduced graphene oxide was subjected to characterization using UV-Visible absorption spectroscopy, X-ray diffraction (XRD), Fourier Transform Infra-Red (FTIR) spectroscopy and Scanning Electron Microscopy (SEM) with Energy Dispersive X-ray (EDX) analysis.
Fish and the experimental conditions
Adult zebrafish were obtained from a commercial breeding centre in Chennai, Tamil Nadu, India. One week prior to the trial, fish were acclimated to the aquatic facility’s dechlorinated tap water. The photoperiod (12L:12D) and the water temperature (26.0±1.0°C) were maintained at steady levels. Daily readings of the water’s pH and dissolved oxygen concentration were taken. Two times a day, fish were given commercial feed.
Experimental design
Fish were randomly classified into 8 groups. Each group comprised 6 animals in 3 L of dechlorinated tap water (2 fish/L). Each group was treated as below:
Group 1: Control, Group 2: rGO (10 mg/L), Group 3: Rutin (50 mg/L), Group 4: rGO (10
mg/L) + Rutin (50 mg/L), Group 5: Rutin (100 mg/L), Group 6: rGO (10 mg/L) + Rutin (100 mg/L),
Group 7: Rutin (200 mg/L), Group 8: rGO (10 mg/L) + Rutin (200 mg/L).
The H2O, rGO NPs, and rutin administered to both the control and treated groups were rehabilitated daily. Each group was subjected to the same treatment mentioned above for 14 days.
Behavioural analysis
The T-maze is a multi-species operative task used to evaluate memory and investigate the learning and memory processes of zebrafish. The Novel Tank Test (NTT) examined the anxiety- like behaviour and changes in the exploratory behaviour of zebrafish. The Light/Dark Preference Test (LDT) analyzed the anxiety and exploratory behaviour of zebrafish in the presence of a motivational conflict between light and dark sleeves. The T-maze, NTT, and LDT experiments were carried out following the procedures outlined in the protocol provided by Devaraj et al.28.
Sample Collection
After 14 days, the fish were submerged in ice-cold water and sacrificed. The liver was carefully dissected and rinsed twice with PBS. Subsequently, the tissue was stored at -80 °C for future analysis. Prior to homogenization, the liver was washed with an ice-cold saline solution. Homogenization was performed using a chilled mortar and pestle with 1 mL of homogenization buffer at pH 7.4 (composed of 50 mM Tris-HCl buffer with 1 mM EDTA and 0.25 mM sucrose). The resulting homogenate was subsequently centrifuged at 10,000 rpm for 15 minutes at 4°C, and the supernatant was collected for further investigation.
Analyses of Biochemical Parameters
The supernatant obtained from the tissue homogenate was utilized to assess various biochemical parameters. Lipid peroxidation (LPO), an indicator of oxidative stress, was estimated based on the protocol defined by Devasagayam and Tarachand29. Superoxide dismutase (SOD) activity was determined using the protocol established by Marklund and Marklund30, while catalase (CAT) activity was estimated following the protocol developed by Aebi31. Glutathione peroxidase (GPx) activity was estimated using the procedure defined by Rotruck et al.32. Reduced glutathione (GSH) levels were analyzed as stated by the protocol outlined by Ellman33. Glutathione reductase (GR) activity was assessed based on the procedure developed by Calberg and Mannervik34. Glutathione S-transferase (GST) activity was estimated following the technique established by Habig et al.35. Lastly, the concentration of vitamin C was determined using the procedure described by Roe and Kuether36.
Histological examination
The liver of the adult fish was removed, thoroughly cleaned in 0.9% saline, then preserved in 10% neutral buffered formalin, embedded in low melting point paraffin (56°C), and sectioned (5 µm). Sections underwent deparaffinization, graded ethanol dehydration for several hours, H&E staining, and microscopy examination (Nikon Eclipse 50i).
Statistical Analysis
The data are stated as mean ± standard error of means (SEM) and encompass the entire dataset. To compare the outcomes among the various groups, a one-way analysis of variance (ANOVA) was conducted, followed by a Duncan’s Multiple Range Test (DMRT). SPSS version
Software package was employed for the statistical A significance level of p < 0.05 was employed to assess the statistical significance.
Results
Characterization of rGO NPs UV-Visible spectroscopy
The optical characteristics of nanoparticles were evaluated using the UV-Vis absorption spectrum. The absorption spectrum was captured, between 200 and 800 nm. The maximum absorption peak of GO appears at 230 nm and rGO was discovered at 278 nm as shown in Fig. 1 (a & b)
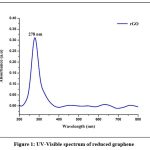 |
Figure 1: UV-Visible spectrum of reduced graphene oxide |
X-Ray diffraction (XRD)
The average crystallite size of rGO nanoparticles was analyzed with the XRD technique. The XRD pattern of produced rGO nanoparticles is depicted in Fig. 2. The main strong peak of rGO was observed at 26.4° which corresponds to the plane (002). It matched the peak described by Pratima et al. [43] and so the targeted nanoparticle size was confirmed. The Scherrer’s formula in the following equation, D = (0.94λ)/(βcosθ) was used to determine the particle size based on the width of XRD peaks. The calculated crystal size measured at 12.4 nm.
 |
Figure 2: XRD pattern of reduced graphene oxide |
Fourier transform infrared spectroscopy (FTIR)
The targeted nanoparticles existing functional groups are identified using Fourier transform infrared spectroscopy. Fig. 3 depicts the rGO FTIR spectra. The peak at 3796 cm-1 is attributed to O-H stretching vibration, while a peak in the region 3686 cm-1 may be due to O-H stretching. The peak at 2923 cm-1 is attributed to C-H stretching, and the peak at 1752 cm-1 corresponds to C=O stretching. The peak at 1592 cm-1 corresponds to C=C stretching. The peak at 1454 cm-1 is attributed to C-H bending, and the peak at 1156 cm-1 corresponds to C-N stretching. Finally, the peak at 857 cm-1 is attributed to C-H bending vibration, which predicts the targeted nanoparticle. During the thermal reduction process, the elimination of O2 resulted in a reduction in the spectrum size and a substantial decrease in oxygen-containing functional groups.
 |
Figure 3: FTIR spectra of reduced graphene oxide |
Scanning Electron Microscopy (SEM) with Energy Dispersive X-ray (EDX)
Scanning Electron Microscopy (SEM) is a powerful method for generating insightful data for describing nanostructures. The SEM was utilized to assess the morphology of the synthesized rGO NPs, which provided significant magnification of the material’s surface. Fig. 4 (a & b) describes the micrographs of rGO NPs with diverse magnifications, which demonstrate individual thin sheet-like structures, randomly aggregated with distinct edges through self-assembly techniques demonstrating the successful reduction of GO into rGO.
The EDX analysis indicates that the synthesized NPs contain elemental mass % C (86.14 ± 0.21) and O (13.86 ± 0.31), endorsing its higher purity without any impurities. Further, the EDX analysis confirms that the synthesized NPs are rGO. Fig. 5 represents the EDX analysis and the details are shown in Table 1.
 |
Figure 4: (a – b) Micrographs of rGO NPs with different magnifications |
Table 1: Energy Dispersive X-ray measurements of reduced graphene oxide.
| Element | Line | Mass% | Atom% |
| C | K | 86.14 ± 0.21 | 89.22 ± 0.22 |
| O | L | 13.86 ± 0.31 | 10.78 ± 0.24 |
| Total | 100.00 | 100.00 | |
| Fitting ratio 0.3665 |
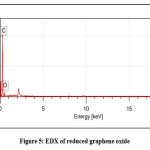 |
Figure 5: EDX of reduced graphene oxide |
Behavioural analysis of Danio rerio
The behavioural tests, including T-maze, NTT and LDT significantly analyzed the adult zebrafish’s learning, memory, anxiety and exploratory behaviours. The fish in our study subjected to rGO showed exceptionally dull behaviour compared to the control, rutin alone and rGO + rutin groups. Rutin significantly reduced the behavioural alterations caused by the rGO in the rGO + rutin groups. Thus, behavioural improvement could be observed in the rGO + rutin-treated groups compared to the rGO alone treated zebrafish groups.
T-maze for learning and memory
The total time spent on the green and red arm of the T-maze and the number of entries to the green and red arm of the T-maze by the control, rGO, rutin, rGO + rutin treated groups have been graphically represented in Fig. 6 (a & b).
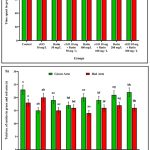 |
Figure 6: T-maze (a) Graphs depicting the time spent in the red and green arms of the T-maze by the control, rGO, rutin, rGO + rutin supplemented groups. |
Novel Tank Test (NTT) for checking anxiety-induced behavioural changes
The total time spent in the top and bottom zones of the tank and the number of entries to the top and bottom zones by the control, rGO, rutin, rGO + rutin treated groups are graphically represented in Fig. 7 (a & b).
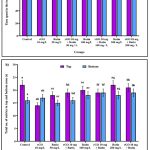 |
Figure 7: NTT (a) Graphs depicting the time spent in the top and bottom zone of the tank by the control, rGO, rutin, rGO + rutin supplemented groups. |
Light and Dark Test (LDT) for assessing anxiety-like behavioural changes
The total time spent in the light and dark compartments and the total number of entries to the light and dark compartments by the control, rGO, rutin, rGO + rutin treated groups are graphically represented in Fig. 8 (a & b).
 |
Figure 8: LDT (a) Graphs depicting the time spent in the light and the dark zone by the control, rGO, rutin, rGO + rutin supplemented groups. |
Oxidative stress markers
The adult zebrafish gill tissue was examined to assess the impact of rGO NPs and rutin on oxidative stress markers. The groups that received only rGO (10mg/L) and those subjected to the combination of rGO with the lowest dose of rutin (50mg/L) exhibited a significant decrease in antioxidants, including SOD (Fig. 9a), CAT (Fig. 9b), GPx (Fig. 9c), GSH (Fig. 9d), GR (Fig. 9e), GST (Fig. 9f), and Vit C (Fig. 9g), compared to the control and rutin-only treated groups (50mg/L, 100mg/L, and 200mg/L). A steady increase in the oxidant level LPO (Fig. 9h) was detected in the rGO NPs treated zebrafish compared to the control and rutin alone groups. In addition, the antioxidant levels in the rGO + rutin treated groups (different doses as mentioned above) were elevated as compared to rGO alone treated fish in a dose-dependent manner. However, the markers exhibited significant changes in response to varying doses with the decrease in the antioxidant levels. This suggests that the treated groups encountered elevated oxidative stress.
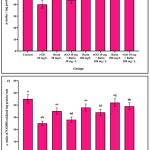 |
Figure 9: The oxidative stress markers such as SOD (a), CAT (b), GPx (c), GSH (d), GR (e), GST (f), Vit C (g) and LPO (h) in the gill tissue of control, rGO, rGO + rutin supplemented groups. |
Histology
Fig.10 (a-h) depicts the morphological analysis of adult zebrafish liver tissue from both the control and treated groups. The liver samples of the control group and rutin (50 mg/L, 100 mg/L and 200 mg/L) exposed zebrafish showed normal morphological structure. Histological abnormalities were found in the liver tissues treated with rGO (10 mg/L), with clear evidence of sinusoidal and venous congestion, cytoplasmic vacuolation of hepatocytes, necrosis and increased hepatic plate gap. However, zebrafish treated with rGO and rutin supplementation (distinct doses mentioned above) showed markedly reduced changes, in the irregular shape of hepatocytes and cytoplasmic vacuolation of hepatocytes. Less harm was demonstrated in the groups treated with rGO along with rutin.
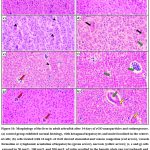 |
Figure 10: Morphology of the liver in adult zebrafish after 14 days of rGO nanoparticles and rutin exposure. |
Discussion
Carbon nanomaterials have garnered significant attention in numerous studies owing to their remarkable mobility as charge carriers, high thermal conductivity, and advantageous electronic and mechanical properties. These materials find applications in various fields, including electrochemical devices, catalysis, energy storage, cell imaging, photothermal therapy, biosensors, and drug delivery37,38. The two most widely used and produced nanoparticles are rGO and GO. Due to structural modifications, rGO performs better than GO in activity, biocompatibility, dispersion, and binding sites39-42.
As a model organism, zebrafish (Danio rerio) are frequently utilized in scientific research because of their genetic similarity to humans, rapid reproduction, and transparent embryos that make it simple to see internal organs. They are accommodative in researching the toxic effects of various substances, such as pharmaceutical compounds and environmental pollutants.
Most of the research has been concentrated on the impacts of graphene-based materials on zebrafish’s embryonic and larval stages, so there are few toxicology studies involving adult zebrafish and rGO. According to Pratima et al.,43, rGO toxicity significantly impaired behavioural responses and disturbed antioxidant levels. In addition, zebrafish exposed to a sub-lethal dose of rGO (10 mg/L) experienced oxidative stress, metabolic abnormalities, and immune responses.
This study examined the potential ameliorative effects of rutin against the sublethal dose (10 mg/L) of rGO-induced toxicity in adult zebrafish. To assess the protective impact of rutin, zebrafish were treated with various concentrations of rutin (50 mg/L, 100 mg/L, and 200 mg/L) over 14 days, while rGO was administered during the same timeframe. The fish underwent various behavioural, biochemical, and histological examinations following the study period.
Exposure to rGO has been associated with behavioural and physiological changes in fish. Fish exposed to graphene-based materials exhibited changes in swimming behaviour, feeding patterns, and reproductive parameters. These effects may disrupt normal ecological interactions and reduce the population’s fitness 44. By examining LDT, NTT, and T-maze tests, this research reveals that rutin supplementation effectively countered the behavioural modifications induced by rGO administration in zebrafish. For instance, in the LDT, the zebrafish exhibited increased movement towards the brightly lit zone and spent more time in the light zone compared to the group that received only rGO. NTT demonstrated that the co-supplementation of rutin after exposure to rGO significantly altered the bottom-dwelling behaviour of zebrafish compared to the other groups. T maze revealed that rutin treatment also improves the zebrafish’s impaired learning and memory caused by rGO exposure.
Fish have been shown to exhibit signs of oxidative stress when exposed to RGO. Reactive oxygen species (ROS) are produced in excess compared to an organism’s detoxification capacity, which results in oxidative stress. Excessive ROS can impact the organism’s overall health because they can cause cellular damage and inflammation45.
In response to ROS, zebrafish activate an antioxidant defence mechanism similar to mammals46. Antioxidants act as the foremost line of defence against oxidative stress, effectively scavenging free radicals to safeguard cells from potential damage47,48. When the antioxidant defence system fails to neutralize excessive ROS, it results in the inactivation of enzymes. One potential cause of this inactivation is the overproduction of ROS. To counteract this, Superoxide Dismutase (SOD) plays a vital role by converting oxygen-free radicals into hydrogen peroxide (H2O2). Subsequently, Catalase (CAT) and Glutathione Peroxidase (GPx) work together to break down H2O2 into water (H2O) and oxygen (O2) molecules. This enzymatic process is crucial for maintaining the intracellular redox balance49. However, along with the observed inhibition of SOD and CAT activities, the activity of GPx was also reduced50. Therefore, reducing the activities of these essential primary antioxidants could lead to the accumulation of H2O2 and its degradable products51.
Sublethal exposure to rGO resulted in a dysfunction of the essential primary antioxidant defence system and a decrease in SOD, CAT, and GPx activity in the gill tissues of zebrafish. This may be due to an excess of superoxide ion production. However, the antioxidant levels of the groups supplemented with the combination of rGO and rutin showed some improvement. This could be due to rutin’s protective effect against rGO.
The glutathione system, encompassing the non-enzymatic antioxidant defence, is the secondary line of protection against oxidative damage. GSH and its associated enzymes, GR and GST, play vital roles within this system. GSH, the predominant non-protein thiol found in cells, is a scavenger of free radicals and a substrate for GPx and GST. The regeneration of GSH, crucial for combating oxidative stress, heavily relies on the activity of GR 51. GR transforms oxidized glutathione (GSSG) to its reduced form (GSH). In its reduced state, glutathione regulates ROS within the cell. Conversely, GST catalyzes the conjugation of GSH with various xenobiotic substrates to facilitate detoxification52.
Additionally, GSH helps maintain other antioxidants like vitamins E and C in their active, reduced state within the cellular environment. Its depletion serves as a significant biomarker of oxidative stress in animals and humans53. In a previous study by Lushchak54, zebrafish exposed to CuO nanoparticles displayed a notable reduction in GSH levels. Another non- enzymatic antioxidant, vitamin C, acts as an oxidative stress inhibitor through various mechanisms. It scavenges reactive oxygen species by trapping radicals in the aqueous phase and halting peroxidation, thereby safeguarding cell membranes against oxidative damage55. Zhang et al.56 reported significantly lower levels of vitamin C in fish exposed to CuO-NPs compared to the control group.
However, in our research, the gill tissue of adult zebrafish treated solely with rGO and a combination of rGO and rutin (50mg/L) showed reduced GSH, GR, GST and vitamin C activities. At the same time, rGO and rutin supplementation at higher doses (100 mg/L and 200 mg/L) showed better activities than rGO alone groups.
Lipid peroxidation (LPO) is a process where polyunsaturated fatty acids (PUFAs) undergo oxidative degradation. It plays a vital role in maintaining membrane function, structural integrity, and the deactivation of certain membrane-bound enzymes57. Excessive ROS can induce LPO, damaging tissue. However, the presence of antioxidant and detoxification systems can help mitigate LPO by eliminating excess ROS [49]. Malondialdehyde (MDA) is commonly utilized as a biomarker to assess the level of LPO and indicates oxidative damage or stress58.
Our study revealed that the rGO alone groups exhibited higher levels of LPO than the control group. In contrast, the rGO and rutin-treated groups demonstrated lower levels of LPO than the rGO-treated group alone. These findings suggest that rutin treatment may help to mitigate LPO. Moreover, Wang et al.59 propose that SOD is the first-line protective enzyme against ROS and LPO. Therefore, a decline in SOD activity may lead to an increase in LPO.
Histological abnormalities including sinusoidal and venous congestion, cytoplasmic vacuolation of hepatocytes, increased hepatic plate gap and necrosis were observed in the liver tissues of rGO alone (10 mg/L) treated group. Nevertheless, zebrafish treated with rGO along with rutin supplementation (50 mg/L, 100 mg/L and 200 mg/L) showed markedly reduced changes like irregular shape of hepatocytes and cytoplasmic vacuolation of hepatocytes. The histopathological changes observed in our study were consistent with the findings stated by Rajini et al. 60, Liu et al. 61, and Sezgi Arman et al. 62 in their respective investigations on zebrafish liver.
Conclusion
According to the results of this research, exposure to a sublethal dose of rGO (10 mg/L) led to oxidative stress in zebrafish, resulting in reduced antioxidant levels. In addition, it led to behavioural changes, liver tissue morphological alterations and elevated LPO levels in the adult zebrafish. However, the alterations were markedly decreased in the groups treated with rutin and rGO in combination. After exposure to rGO, rutin exhibits a therapeutic ability to scavenge ROS and hydroxy radicals effectively. The desirable quantity of rutin supplementation for alleviating the detrimental effects of rGO exposure falls within the range of 100 and 200 mg/L. Further study is essential to entirely comprehend the potential toxicity of rGO in adult zebrafish and evaluate its long-term effects on individual and population levels. It is also essential to consider the potential environmental impacts of rGO release and its accumulation in aquatic ecosystems. This study would help to investigate the safety and potential risks associated with graphene-based materials and ensure their responsible usage in various applications.
Acknowledgement
The authors would like to acknowledge the Department of Biochemistry and Biotechnology, Annamalai University for providing the laboratory facilities and all other required consumables and equipment.
Conflict of Interest
The authors declare no conflict of interest to disclose.
Funding Sources
There are no funding sources
Author Contributions
The authors confirm contribution to the paper as follows: study conception and design: Briska
Jifrina Premnath; data collection: Manoj Kumar Srinivasan; analysis and interpretation of results:
Briska Jifrina Premnath; draft manuscript preparation: Nalini Namasivayam. All authors
reviewed the results and approved the final version of the manuscript.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Ethical Statement
Since the work was done before the CCSEA decision on fish ethics. There are no ethical issues
involved to carry out the research.
References
- Oliveira, E.F., Braga, G.B., Tarley, C.R.T. and Pereira, A.C. Thermally reduced graphene oxide: synthesis, studies and characterization. Journal of Materials Science. 2018; 53(17), pp.12005-12015.
CrossRef - Kosowska, K., Domalik-Pyzik, P., Krok-Borkowicz, M. and Chłopek, J. Synthesis and characterization of chitosan/reduced graphene oxide hybrid composites. Materials. 2019; 12(13), pp.2077.
CrossRef - Kosowska, K., Domalik-Pyzik, P., Nocuń, M. and Chłopek, J. Chitosan and graphene oxide/reduced graphene oxide hybrid nanocomposites–Evaluation of physicochemical Materials Chemistry and Physics. 2018; 216, pp.28-36.
CrossRef - Bianco, A., Cheng, H.M., Enoki, T., Gogotsi, Y., Hurt, R.H., Koratkar, N., Kyotani, T., Monthioux, M., Park, C.R., Tascon, J.M. and Zhang, J. All in the graphene family–A recommended nomenclature for two-dimensional carbon Carbon. 2013; 65, pp.1-6.
CrossRef - Wang, Y., Li, Y., Tang, L., Lu, J. and Li, J. Application of graphene-modified electrode for selective detection of dopamine. Electrochemistry communications. 2009; 11(4), pp.889-
CrossRef - Sun, Y., Huang, K.J., Wei, S.Y., Wu, Z.W. and Ren, F.P. A graphene-based electrochemical sensor for sensitive determination of caffeine. Colloids and Surfaces B: Biointerfaces. 2011; 84(2), pp.421-426.
CrossRef - Kaur, M., Kaur, H. and Kukkar, D. Synthesis and characterization of graphene oxide using modified Hummer’s method. In AIP conference proceedings. AIP Publishing LLC. 2018; pp. 030180.
CrossRef - Bahrami, S., Baheiraei, N., Mohseni, M., Razavi, M., Ghaderi, A., Azizi, B., Rabiee, N. and Karimi, M. Three-dimensional graphene foam as a conductive scaffold for cardiac tissue Journal of biomaterials applications. 2019; 34(1), pp.74-85.
CrossRef - Bharathi, D., Ranjithkumar, R., Chandarshekar, B. and Bhuvaneshwari, V. Bio-inspired synthesis of chitosan/copper oxide nanocomposite using rutin and their anti-proliferative activity in human lung cancer cells. International Journal of Biological Macromolecules. 2019; 141, 476-483.
CrossRef - Wu, , Chen, J., Fan, L.M., Liu, K., Zhang, N., Li, S.W., Zhu, H. and Gao, H.C. Analysis of the effect of rutin on GSK-3β and TNF-α expression in lung cancer. Experimental and therapeutic medicine. 2017; 14(1), pp.127-130.
CrossRef - Gullon, B., Lú-Chau, T.A., Moreira, M.T., Lema, J.M. and Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its Trends in food science & technology. 2017; 67, pp.220-235.
CrossRef - Hoai, T.T., Yen, P.T., Dao, T.T.B., Long, L.H., Anh, D.X., Minh, L.H., Anh, B.Q. and Thuong, T. Evaluation of the cytotoxic effect of rutin prenanoemulsion in lung and colon cancer cell lines. Journal of Nanomaterials. 2020; pp.1-11.
CrossRef - Lin, , Zhao, Y., Nel, A.E. and Lin, S. Zebrafish: an in vivo model for nano EHS studies. Small. 2013; 9(9‐10), pp.1608-1618.
CrossRef - Jiang, D., Chen, Y., Li, N., Li, W., Wang, Z., Zhu, J., Zhang, H., Liu, B. and Xu, S. Synthesis of luminescent graphene quantum dots with high quantum yield and their toxicity study. PLoS One. 2015; 10(12), pp.e0144906.
CrossRef - Mu, J., Hou, C., Wang, H., Li, Y., Zhang, Q. and Zhu, M. Origami-inspired active graphene- based paper for programmable instant self-folding walking devices. Science advances. 2015; 1(10), pp.e1500533.
CrossRef - Wang, G., Rong, Z.H.O.U., Jiang, D., Jing, E.S., Qian, X.U., Jing, S.I., Chen, Y.P., Xin, Z., Lu, G.A.N., Li, J.Z. and Zhang, H. Toxicity of graphene quantum dots in zebrafish embryo. Biomedical and Environmental Sciences. 2015; 28(5), pp.341-351.
- Ren, C., Hu, X., Li, X. and Zhou, Q. Ultra-trace graphene oxide in a water environment triggers Parkinson’s disease-like symptoms and metabolic disturbance in zebrafish Biomaterials. 2016; 93, pp.83-94.
CrossRef - Li, A., Zhang, C. and Zhang, C. and Zhang, Y.F. Thermal conductivity of graphene-polymer composites: Mechanisms, properties, and applications. Polymers. 2017; 9(9), p.437.
CrossRef - Souza, P., Baretta, J.F., Santos, F., Paino, I.M. and Zucolotto, V. Toxicological effects of graphene oxide on adult zebrafish (Danio rerio). Aquatic toxicology. 2017; 186, pp.11-18.
CrossRef - Tabish, T.A., Pranjol, M.Z.I., Hayat, H., Rahat, A.A., Abdullah, T.M., Whatmore, J.L. and Zhang, S. In vitro toxic effects of reduced graphene oxide nanosheets on lung cancer cells. Nanotechnology. 2017; 28(50), p.504001.
CrossRef - Tabish, T.A., Zhang, S. and Winyard, P.G. Developing the next generation of graphene- based platforms for cancer therapeutics: The potential role of reactive oxygen Redox biology. 2018; 15, pp.34-40.
CrossRef - Lategan, K., Alghadi, H., Bayati, M., de Cortalezzi, M.F. and Pool, E. Effects of graphene oxide nanoparticles on the immune system biomarkers produced by RAW 264.7 and human whole blood cell cultures. Nanomaterials. 2018; 8(2), p.125.
CrossRef - Ou, L., Song, B., Liang, H., Liu, J., Feng, X., Deng, B., Sun, T. and Shao, L. Toxicity of graphene-family nanoparticles: a general review of the origins and mechanisms. Particle and fibre 2016; 13(1), pp.1-24.
CrossRef - Zhu, X., Zhu, L., Li, Y., Duan, Z., Chen, W. and Alvarez, P.J. Developmental toxicity in zebrafish (Danio rerio) embryos after exposure to manufactured nanomaterials: buckminsterfullerene aggregates (nC60) and Environmental Toxicology and Chemistry: An International Journal. 2007; 26(5), pp.976-979.
CrossRef - Zhang, , Ali, S.F., Dervishi, E., Xu, Y., Li, Z., Casciano, D. and Biris, A.S. Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma- derived PC12 cells. ACS nano. 2010; 4(6), pp.3181-3186.
CrossRef - Yan, L., Wang, Y., Xu, X., Zeng, C., Hou, J., Lin, M., Xu, J., Sun, F., Huang, X., Dai, L. and Lu, Can graphene oxide cause damage to eyesight?. Chemical research in toxicology. 2012; 25(6), pp.1265-1270.
CrossRef - Cao, and Zhang, Y. Study of reduced graphene oxide preparation by Hummers’ method and related characterization. Journal of Nanomaterials. 2015; pp.2-2.
CrossRef - Devaraj, U., Shanmugasundaram, T., Ramu, A. and Balamurugan, E. A Study on the Alleviating Effects of N-phthaloyl-γ-Aminobutyric Acid (P-GABA) on the Behavioral, Histopathological and Biochemical Adverse Effects of Sleep Deprivation in Zebrafish (Danio rerio). Journal of Pharmaceutical Research International. 2021; 33(60A), 580- 596.
CrossRef - Devasagayam, P. and Tarachand, U. Decreased lipid peroxidation in the rat kidney during gestation. Biochemical and biophysical research communications. 1987; 145(1), pp.134- 138.
CrossRef - Marklund, and Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European journal of biochemistry. 1974; 47(3), pp.469-474.
CrossRef - Aebi, H. [13] Catalase in vitro. In Methods in enzymology. 1984; (Vol. 105, pp. 121-126). Academic
CrossRef - Rotruck, T., Pope, A.L., Ganther, H.E., Swanson, A.B., Hafeman, D.G. and Hoekstra, W. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973; 179(4073), pp.588-590.
CrossRef - Ellman, L. Tissue sulfhydryl groups. Archives of biochemistry and biophysics. 1959; 82(1), pp.70-77.
CrossRef
- Carlberg, and Mannervik, B. [59] Glutathione reductase. In Methods in enzymology. Academic press. 1985; (Vol. 113, pp. 484-490).
- Habig, H., Pabst, M.J. and Jakoby, W.B. Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. Journal of biological Chemistry. 1974; 249(22), pp.7130-7139.
CrossRef - Roe, J.H. and Kuether, C.A. The determination of ascorbic acid in whole blood and urine through the 2, 4-dinitrophenylhydrazine derivative of dehydroascorbic acid. Journal of Biological chemistry. 1943; 147, pp.399-407.
CrossRef - Liu, X., Wang, M., Zhang, S. and Pan, B. Application potential of carbon nanotubes in water treatment: a Journal of Environmental Sciences. 2013; 25(7), pp.1263-1280.
CrossRef - Lonkar, P., Deshmukh, Y.S. and Abdala, A.A. Recent advances in chemical modifications of graphene. Nano Research. 2015; 8, pp.1039-1074.
CrossRef - Bayunova, , Barannikova, I. and Semenkova, T. Sturgeon stress reactions in aquaculture. Journal of Applied Ichthyology. 2002; 18(4‐6), pp.397-404.
CrossRef - Balandin, A.A., Ghosh, S., Bao, W., Calizo, I., Teweldebrhan, D., Miao, F. and Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano letters. 2008; 8(3), pp.902-907
CrossRef - Chen, H., Jang, C., Ishigami, M., Xiao, S., Cullen, W.G., Williams, E.D. and Fuhrer, M.S. Diffusive charge transport in graphene on SiO2. Solid State Communications. 2009; 149(27-28), pp.1080-1086.
CrossRef - Lee, D.Y., Khatun, Z., Lee, J.H., Lee, Y.K. and In, I. Blood compatible graphene/heparin conjugate through noncovalent Biomacromolecules. 2011; 12(2), pp.336-341.
CrossRef - Pratima, B.J., Ragunath, R. and Nalini, N. Protective effect of carvacrol on biochemical, immunological and gill morphological induction through reduced graphene oxide (RGO) exposure on zebrafish (Danio rerio). International Journal of Entomology Research. 2022; 7(1), 20-29.
- Li,D , Wang, G., Du, L., Zheng, Y. and Wang, Z. Recent advances in intelligent recognition methods for fish stress behavior. Aquacultural Engineering. 2022; 96, p.102222.
CrossRef - Pandey, , Parvez, S., Sayeed, I., Haque, R., Bin-Hafeez, B. and Raisuddin, S. Biomarkers of oxidative stress: a comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Science of the total environment. 2003; 309(1-3), pp.105-115.
CrossRef - Zhu, , Sun, L., Liu, M. and Zhou, J. Effect of nitric oxide on reactive oxygen species and antioxidant enzymes in kiwifruit during storage. Journal of the Science of Food and Agriculture. 2008; 88(13), pp.2324-2331.
CrossRef - Halliwell, B. and Gutteridge, J.M. Free radicals in biology and medicine. Oxford university press, USA. 2015.
CrossRef - Melegari, S.P., Perreault, F., Moukha, S., Popovic, R., Creppy, E.E. and Matias, W.G. Induction to oxidative stress by saxitoxin investigated through lipid peroxidation in Neuro 2A cells and Chlamydomonas reinhardtii Chemosphere. 2012; 89(1), pp.38-43.
CrossRef - Ni, H., Peng, L., Gao, X., Ji, H., Ma, J., Li, Y. and Jiang, S. Effects of maduramicin on adult zebrafish (Danio rerio): Acute toxicity, tissue damage and oxidative Ecotoxicology and environmental safety. 2019; 168, pp.249-259.
CrossRef - Gyimah, , Dong, X., Qiu, W., Zhang, Z. and Xu, H., Sublethal concentrations of triclosan elicited oxidative stress, DNA damage, and histological alterations in the liver and brain of adult zebrafish. Environmental Science and Pollution Research. 2020; 27, pp.17329- 17338.
CrossRef - Wu, M., Xu, H., Shen, Y., Qiu, W. and Yang, M. Oxidative stress in zebrafish embryos induced by short‐term exposure to bisphenol A, nonylphenol, and their Environmental toxicology and chemistry. 2011; 30(10), pp.2335-2341.
CrossRef - Ganesan, K., Sukalingam, K. and Xu, B. Solanum trilobatum L. ameliorate thioacetamide- induced oxidative stress and hepatic damage in albino rats. Antioxidants. 2017; 6(3), p.68.
CrossRef - Mousavi, R., Jafari, M., Rezaei, S., Agha-Alinejad, H. and Sobhani, V. Evaluation of the effects of different intensities of forced running wheel exercise on oxidative stress biomarkers in muscle, liver and serum of untrained rats. Lab animal. 2020; 49(4), pp.119- 125.
CrossRef - Lushchak, I. Glutathione homeostasis and functions: potential targets for medical interventions. Journal of amino acids. 2012.
CrossRef - Xiang, Q., Xu, B., Ding, Y., Liu, X., Zhou, Y. and Ahmad, F. Oxidative stress response induced by butachlor in zebrafish Embryo/Larvae: The protective effect of vitamin C. Bulletin of environmental contamination and toxicology. 2018; 100, pp.208-215.
CrossRef - Zhang, , An, C., Gao, Y., Leak, R.K., Chen, J. and Zhang, F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Progress in neurobiology. 2013; 100, pp.30-47.
CrossRef - GÖKÇE, and ÜÇÜNCÜ, S.İ. Biochemical evaluations of Quinalphos exposed zebrafish liver organotypic tissue culture. Journal of Scientific Perspectives. 2019; 3(4), pp.319-328.
CrossRef - Chen, L., Sun, Y.L., Liu, Z.H. and Li, Y.W. Sex-dependent effects of subacute mercuric chloride exposure on histology, antioxidant status and immune-related gene expression in the liver of adult zebrafish (Danio rerio). Chemosphere. 2017; 188, pp.1-9.
CrossRef - Wang, H., Zhong, X., Shi, W.Y. and Guo, B. Study of malondialdehyde (MDA) content, superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities in chickens infected with avian infectious bronchitis African Journal of Biotechnology. 2011; 10(45), pp.9213-9217.
CrossRef - Rajini, , Revathy, K. and Selvam, G. Histopathological changes in tissues of Danio rerio exposed to sub lethal concentration of combination pesticide. Indian J. Sci. Technol. 2015; 8, pp.1-12.
CrossRef - Liu, W., Huang, G., Su, X., Li, S., Wang, Q., Zhao, Y., Liu, Y., Luo, J., Li, Y., Li, C. and Yuan, D. Zebrafish: a promising model for evaluating the toxicity of carbon dot-based ACS Applied Materials & Interfaces. 2020; 12(43), pp.49012-49020.
CrossRef - Arman, S. Effects of acute triclosan exposure on gill and liver tissues of zebrafish (Danio rerio). In Annales De Limnologie-International Journal of Limnology. EDP Sciences. 2021; (Vol. 57, p. 6).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





