Manuscript accepted on : 25-12-2023
Published online on: 07-02-2024
Plagiarism Check: Yes
Reviewed by: Dr Maysaa Kadhim Al-Malkey
Second Review by: Dr. Viswanath Vittaladevaram
Final Approval by: Dr. Ali Mohamed Elshafei
Jhanani Gopalraaj and Krishnakumar Velayudhannair*
and Krishnakumar Velayudhannair*
Department of Life Sciences, CHRIST (Deemed to be University), Bangalore Central Campus, Hosur Main Road, Dharmaram Post, Bengaluru, Karnataka, India.
Corresponding Author E-mail: krishnakumar.v@christuniversity.in
DOI : http://dx.doi.org/10.13005/bbra/3209
ABSTRACT: The introduction of protease enzyme supplementation in the early stages of fish can address protease deficiency, thereby promoting increased protein digestion and overall organismal well-being. This study focuses on evaluating the influence of Ananas comosus peel extract (AcPE) on the growth, biochemical profile, and haematological characteristics of Labeo rohita fingerlings. Over a 45 day period, L. rohita fingerlings were fed a basal diet enriched with AcPE (at a ratio of 1:2 extract to feed), with observations taken at 15-day intervals. The control group received the basal diet without AcPE. The findings highlight an overall enhancement in growth parameters among the experimental group fingerlings that were fed the AcPE-supplemented diet, with the exception of length gain, when compared to the control group. Examination of the fingerlings' biochemical profiles revealed a significantly higher protein and amino acid content, while carbohydrate and lipid content remained relatively stable within the two groups. In terms of haematological profiles, a noteworthy increase in total red blood cell count and haematocrit value was observed, while white blood cell count and haemoglobin concentration did not exhibit significant variations. This study underscores the potential benefits of incorporating AcPE into the basal diet as an eco-friendly approach for waste management, while simultaneously enhancing the growth and health of L. rohita fingerlings.
KEYWORDS: Ananas comosus; Aquaculture; Haematology; Labeo rohita; Pisciculture; Proteolytic activity
Download this article as:| Copy the following to cite this article: Gopalraaj J, Velayudhannair K. Growth and Health Response to Dietary Supplementation of Pineapple Peel Extract in Labeo rohita (Hamilton, 1822) Fingerlings. Biotech Res Asia 2024;21(1). |
| Copy the following to cite this URL: Gopalraaj J, Velayudhannair K. Growth and Health Response to Dietary Supplementation of Pineapple Peel Extract in Labeo rohita (Hamilton, 1822) Fingerlings. Biotech Res Asia 2024;21(1). Available from: https://bit.ly/3SPnmdi |
Introduction
India stands as the third-largest contributor to global fish production, boasting an 8% share in the industry production.1 Among the diverse aquatic species cultivated, the freshwater carp known as rohu, or Labeo rohita, holds paramount importance globally and contributes over 2.0 Mt annually.2 Nevertheless, the aquaculture sector grapples with the reality that approximately 80% of operational costs are attributed to feed expenses.3 A principal factor driving these costs is the scarcity and expense of fish meal.4 To mitigate this challenge, extensive research has explored alternative plant-based protein sources in feed formulations, like soybean meal, sesame meal, mustard oilcake, linseed, and corn gluten meal.5
However, the anti-nutritional components within these plant-derived proteins have hampered their digestibility and nutrient utilization, making them less efficient than fish meal.6 Enter exogenous protease enzyme supplementation, which offers a viable solution by enhancing the digestibility of protein from alternative sources.7 This becomes particularly crucial at the hatchery level, given that fish fingerlings possess lower quantities of proteolytic enzymes compared to their adult counterparts.8 Several studies have already investigated the role of exogenous enzyme supplementation in augmenting nutrient digestibility,9-12 histology,13 digestive enzyme secretion,14,15 and intestinal topography.15,16 However, the effect of exogenous enzyme supplementation on the biochemical composition of fish remains an underexplored area, deserving of further investigation.
Pineapple, Ananas comosus L. Merr., is extensively cultivated in numerous tropical regions worldwide, with India emerging as one of the foremost pineapple producers on the global stage.17 The pineapple processing industry generates substantial waste, including peel, core, stem, and crown, with peel accounting for the most substantial portion of this waste (29%-40% w/w).18 This underscores the importance of innovative approaches for the efficient utilization of pineapple peel waste. Pineapple peels are notably rich in phenolic compounds, showcasing potent antioxidant properties and proteolytic enzymes.19,20 Our previous studies confirmed the abundance of bromelain in pineapple peel compared to the other parts of the pineapple (crown, flesh, and core)21 and their role as a feed supplement in O. niloticus.11 A study by Sharma et al.11 also showed that bromelain can enhance the digestibility of spirulina-based diet.22 Bromelain boasts a diverse range of applications across the food industry, cosmetics, and as a feed supplement.11, 18
Hence, the present study proposes the effective utilization of peels of A. comosus as a source of proteolytic enzymes in fish feed formulations, representing an eco-friendly approach to waste management. In light of these considerations, the present study aims to evaluate the impact of peel extract of A. comosus supplementation on the growth, biochemical profiles, and haematological parameters of L. rohita fingerlings.
Material and Methods
Procurement of fish fingerlings
Labeo rohita fingerlings, weighing approximately 27.51±1.13g, were procured from the Fisheries Research and Information Centre (FRIC), Hessarghatta, Karnataka, India. These fingerlings were carefully transported to our laboratory in well-oxygenated habitat water to ensure their well-being during the journey. Upon their arrival at the laboratory, the fingerlings were given a week-long acclimatization period to adapt to the laboratory’s environmental conditions.
Preparation of A. comosus peel crude extract
Ripe A. comosus fruits were procured from a local market in Bengaluru. The peels were separated, washed, and homogenized. Ice-cold sodium acetate-acetic acid buffer (0.1 M), at a pH of 5 was employed for homogenization, using a 1:1 (weight to volume) ratio. Following homogenization, the mixtures underwent a filtration process using a muslin cloth and were centrifuged at 1680 g for 20 minutes at a temperature of 4˚C, in accordance with established protocols of 4˚C.24,25 The resultant supernatants were preserved as crude extracts specific to each part of the pineapple.
Experimental feed preparation and proximate analysis
The commercially available feed (basal diet) was fed to the control group, and the basal diet was supplemented with A. comosus peel extract (AcPE) in a 1:2 ratio and was treated as an experimental diet. These pellets were air-dried and used for feeding trials. Proximate analysis was performed for both control and experimental diets. The crude fiber, crude lipid, crude protein, ash, moisture, and nitrogen-free extract were determined using standard protocols26 and the results were tabulated (Table 1).
Table 1: Proximate composition of the control and experimental diets.
| Proximate Composition (%) | Control diet | Experimental diet |
| Moisture | 10.02±0.18 | 10.05±0.05 |
| Crude protein | 21.05±0.15 | 21.20±0.61 |
| Crude lipid | 9.30±0.23 | 9.20±0.18 |
| Crude fibre | 15.08±0.13 | 16.74±0.81 |
| Ash | 11.71±0.52 | 12.56±0.31 |
| Nitrogen-free extract | 31.03 | 24.56 |
Experimental setup
During the acclimation phase, the fingerlings were provided with a basal diet for sustenance. Throughout this entire period, water quality parameters, including temperature (26.16±0.2°C), pH (7.21±0.01), and dissolved oxygen levels (4.50±0.14 mg L-1) were maintained. After the successful acclimatization phase, the L. rohita fingerlings were randomly allocated into two groups: control and experimental groups. These fingerlings were distributed at a stocking density of 25 individuals per tank and subjected to their respective diets for a duration of 45 days, with each diet group having three replicates. Regular sampling was carried out at intervals of 15 days to meticulously assess growth parameters and the biochemical profile of the fingerlings.
Growth parameters
The growth parameters, which include weight gain (WG), feed conversion ratio (FCR), length gain (LG), specific growth rate (SGR), average daily gain (ADG), feed efficiency ratio (FER), and protein efficiency ratio (PER), were determined every 15 days, using the following formula.
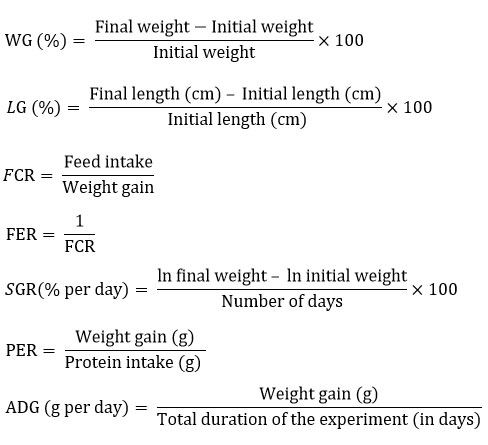
Biochemical parameters
Both control and experimental fingerlings were subjected to biochemical analysis at every 15-day interval. The fingerlings from both control and experimental tanks were randomly selected and the entire organism was homogenized. The total protein,27 total amino acids,28 total lipids,29 and total carbohydrate30 were estimated. All the samples had 5 replicates.
Haematological profiles
To assess the haematological parameters, the blood samples were collected using a sterile syringe from the caudal vein, coated with 2.7% EDTA solution, every 15 days. Haematological parameters such as the total red blood cell (RBC) and white blood cell (WBC) counts (by haemocytometer as described by Rao and Chakrabarti,31 haematocrit (microcentrifuge method), and haemoglobin concentration32 were analysed.
Statistical Analysis
All the obtained data was subjected to one-way ANOVA, using the SPSS software, version 24 (with significance level P<0.05), they were expressed as mean ± SD.
Results and Discussion
Growth Parameters
The growth of fish is dependent on various factors which include the physicochemical properties of rearing water, feed composition, stocking densities and more importantly, feed utilization. Growth analysis is crucial in aquaculture since it helps in estimating the stock sizes, mortality, and feed consumption, and as a health index in the fish population.33 Feed utilisation becomes particularly crucial at the hatchery level, given that fish fingerlings possess lower quantities of proteolytic enzymes compared to their adult counterparts.8 Our study showed that all the growth parameters such as SGR (0.79±0.12 %), WG (78.24±1.17%), PER (4.25±0.57), and FER (0.62±0.22) showed a significant increase amongst there experimental fingerlings, however, FCR (0.42±0.19) decreased significantly in the experimental group (Table 2). The enhanced growth observed in the experimental group could be attributed to the bromelain present in pineapple peel extract. These results were comparable to the findings on C. carpio, O. niloticus, O. mosambicus,11,22,24 which showed an improvement in SGR, FER, WG, PER, and FER on bromelain supplementation. In contrast to our study, Rostika et al.34 reported that FCR values improved significantly on papain supplementation in Iridescent sharks (Pangasianodon hypophthalmus). A significant increase in the overall growth and efficient utilization of nutrients was observed in Juvenile sterlet (Acipenser ruthenus) fed with papain (derived from papaya)35 and actinidin (from kiwifruit).36 Thus, there was an evident increase in the growth performances in A. comosus peel extract-supplemented diet-fed fingerlings, however, the survival rate was not affected (Fig. 1). These results were in accordance with the findings of Subandiyono et al.37 with Java barb, Puntius javanicus and Sharma et al.21 with C. carpio. Additionally, several studies have reported an improved growth performance in fish fed with bromelain and papain in grass carp (Ctenopharyngodon idella),38 papain in Sangkuriang Catfish (Clarias sp)39 and Common carp (C. carpio).40 This overall enhancement of the growth could be supported by the fact that protease enzyme helps in improving nutrient efficiency by reducing or eliminating anti-nutritional factors.41
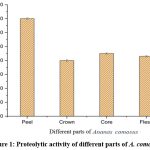 |
Figure 1: Proteolytic activity of different parts of A. comosus. |
Table 2: Growth Parameters of L. rohita fingerlings fed with control and experimental diets on the 30th day.
| Growth Parameters | Control group | Experimental group |
| WG (%) | 21.32±1.18 | 75.24±1.17 |
| LG (%) | 15.01±1.43 | 21.04±1.04 |
| SGR (% day-1) | 0.29±0.13 | 0.79±0.12 |
| FCR | 1.62±0.15 | 0.42±0.19 |
| FER | 0.43±0.16 | 1.82±0.22 |
| PER | 1.13±0.23 | 4.25±0.57 |
| ADG (g day-1) | 0.0029±0.0002 | 0.022±0.002 |
Biochemical profile
The biochemical profile plays a vital role in indicating the growth performance in aquaculture and is dependent on the intrinsic biology and the environmental conditions of the fish.42 In the present study, the total protein, amino acids, and carbohydrates were estimated and presented in L. rohita fingerlings in both control and experimental groups (Table 3). Protein and amino acids play crucial roles in the overall growth performance of the fish.43 It was observed that the total protein and total free amino acids increased significantly in the fingerlings fed with the experimental diet (67.65±2.01 mg g-1 and 44.21±1.54 mg g-1 respectively) compared to control fingerlings (59.01±2.05 mg g-1 and 26.17±1.81 mg g-1 respectively) on day 30. This enhanced digestion in the experimental group fingerlings could be attributed to the proteolytic activity of bromelain present in the peel extract.15 These results were supported by the findings of Gómez-Limia et al.44 and Gopalraaj et al.11 which showed an improvement in protein and amino acids content in fish fed with bromelain supplementation.
Table 3: Biochemical profile of L. rohita fingerlings fed on control and experimental diets.
| Day | Proteins (mg g-1) | Free amino acids (mg g-1) | Carbohydrates (mg g-1) | Lipids (mg g-1) | ||||
| Control | Experimental | Control | Experimental | Control | Experimental | Control | Experimental | |
| 1st | 34.12±2.14 | 34.01±1.02 | 14.23±1.06 | 15.78±1.03 | 15.07±1.55 | 16.08±1.65 | 58.82±1.22 | 58.98±3.02 |
| 10th | 39.73±1.6 | 50.21±1.32 | 16.16±2.06 | 26.14±2.44 | 26.08±1.19 | 27.32±1.42 | 60.21±2.23 | 59.88±2.23 |
| 20th | 44.15±1.46 | 54.72±1.24 | 21.23±0.21 | 34.83±4.34 | 33.93±0.95 | 34.12±2.91 | 60.14±1.56 | 60.31±1.43 |
| 30th | 59.01±2.05 | 67.65±2.01 | 26.17±1.81 | 44.21±1.54 | 36.14±1.82 | 35.23±2.55 | 61.56±2.56 | 61.15±3.16 |
Carbohydrates are important precursors to a few amino acids and nucleic acids and have been shown to improve nutrient utilization in fish45,46. It was observed that the carbohydrate (36.14±1.82 mg g-1 in control groups and 35.23±2.55 mg g-1 in experimental groups) and lipid (61.56±2.56 mg g-1 in control groups and 61.15±3.16 mg g-1 in the experimental group) did not vary significantly between the experimental and control groups). These results were comparable to the results of Sharma et al.21 and Gopalraaj et al.11, which reported that lipid and carbohydrate content remained unaffected on protease supplementation. Hence, it was observed that pineapple peel extract supplementation could help in the overall improvement of the biochemical profile of L. rohita fingerlings.
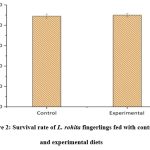 |
Figure 2: Survival rate of L. rohita fingerlings fed with control and experimental diets |
Haematological profile
The haematological profile of fish is an indicator of the health and physiological status of the fish. Several factors that determine a healthy haematological profile include proper water quality, adequate nutrition, and a stress-free environment.47 The haematological profiles of the fish fed with control and experimental diet are presented in Table 4. Accordingly, it was found that AcPE supplementation improved the haematocrit (37.71±0.81%) and red blood cell count (RBC) (5.75±0.11×106 mm-3) in L. rohita fingerlings in the experimental group (Table 4).
These results coincide with the findings of Hassaan et al.48 and Saleh et al.49 stating that RBC count increases with protease supplementation. The increased RBC count is an indicator of better immunity in the fish.47 However, the haemoglobin concentration and WBC count did not vary significantly between the groups. These results were supported by the findings of Adeoye et al.,23 which stated that the WBC count and Haemoglobin levels are unaffected by protease supplementation (Table 4).
Table 4: Haematological profile of L. rohita fingerling fed on control and experimental diets on the 30th day.
| Haematological Parameters | Control group | Experimental group |
| RBCs count x106 mm-3 | 2.66 ± 0.01 | 4.75 ± 0.11 |
| WBCs count x103 mm-3 | 11.09 ± 0.32 | 11.11 ± 0.12 |
| Hb (g lb-1) | 6.88 ± 0.41 | 6.67 ± 0.42 |
| Hematocrit (%) | 33.57 ± 0.61 | 35.71 ± 0.81 |
Conclusion
In conclusion, AcPE supplementation could significantly improve the overall growth performance, biochemical profile, and haematological parameters of L. rohita fingerlings. Thus, the dietary supplementation of AcPE could help boost the aquaculture production of finfish (L. rohita for example) and shellfish, especially in their early growth phases by compensating for the protease deficiency. Our study also promotes the effective utilisation of the pineapple peels thereby helping in waste management. However, the mode of action of protease in improving the haematological parameters is not understood and therefore, future studies demonstrating the relationship between bromelain and haematological parameters are highly warranted.
Acknowledgement
We are grateful to CHRIST (Deemed to be University), Bangalore for extending all the necessary facilities and support for our research.
Authors’ Contribution
Krishnakumar Velayudhannair (KV) conceived the idea, designed the experiments and supervision. Jhanani (JGR) performed the experiments, and data collection and wrote the first draft of the manuscript. KV performed the analysis of the data, revision and final approval of the manuscript All the authors contributed equally to this manuscript and agreed to submit it for publication.
Conflict of Interest
There is no conflict of interest
Funding Sources
This work receives no funding from any agencies.
Data Availability Statement
The manuscript incorporates all datasets produced or examined throughout this research study.
Ethics Approval Statement
Not applicable.
References
- Press Information Bureau. Ministry of Fisheries, Animal Husbandry & Dairying (2021-2022). Government of India. India; 2023.
- The State of World Fisheries and Aquaculture, FAO (2020).
- Naylor R. L., Goldburg R. J., Primavera J. H., Kautsky N., Beveridge M. C. M., Clay J., et al. Effect of aquaculture on world fish supplies. Nature. 2000;405(6790):1017–24.
CrossRef - Tacon A. G. J., Metian M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 2008;285(1–4):146–58.
CrossRef - Kaushik S. J., Covès D., Dutto G., Blanc D. Almost total replacement of fish meal by plant protein sources in the diet of a marine teleost, the European seabass, Dicentrarchus labrax. Aquaculture 2004;230(1–4):391–404.
CrossRef - Francis G., Makkar H. P. S., Becker K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001;199(3–4):197–227.
CrossRef - Yigit N. O., Bahadir Koca S., Didinen B. I., Diler I. Effect of protease and phytase supplementation on growth performance and nutrient digestibility of rainbow trout (Oncorhynchus mykiss, Walbaum) fed soybean meal-based diets. J. Appl. Anim. Res. 2018;46(1):29–32.
CrossRef - Govoni J. J., Boehlert G. W., Watanabe Y. The physiology of digestion in fish larvae. Environ. Biol. Fishes. 1986;16(1–3):59–77.
CrossRef - Lin S., Luo L. Effects of different levels of soybean meal inclusion in replacement for fish meal on growth, digestive enzymes and transaminase activities in practical diets for juvenile tilapia, Oreochromis niloticus × O. aureus. Anim. Feed. Sci. Technol. 2011;168(1–2):80–7.
CrossRef - Drew M. D., Racz V. J., Gauthier R., Thiessen D. L. Effect of adding protease to coextruded flax:pea or canola:pea products on nutrient digestibility and growth performance of rainbow trout (Oncorhynchus mykiss). Anim. Feed. Sci. Technol. 2005;119(1–2):117–28.
CrossRef - Gopalraaj J., Raj J. B. S., Velayudhannair K., Chandrakas L. Bromelain improves the growth, biochemical, and hematological profiles of the fingerlings of Nile Tilapia, Oreochromis niloticus. J. Appl. Biol. Biotechnol. 2022;10:73–77.
CrossRef - Kolkovski S., Tandler A., Izquierdo M. S. Effects of live food and dietary digestive enzymes on the efficiency of microdiets for seabass (Dicentrarchus labrax) larvae. Aquaculture, 1997;148(4):313–22.
CrossRef - Mathlouthi N., Lallès J. P., Lepercq P., Juste C., Larbier M. Xylanase and β-glucanase supplementation improve conjugated bile acid fraction in intestinal contents and increase villus size of small intestine wall in broiler chickens fed a rye-based diet. J. Anim. Sci. 2002;80(11):2773–9.
CrossRef - Lin S., Mai K., Tan B. Effects of exogenous enzyme supplementation in diets on growth and feed utilization in tilapia, Oreochromis niloticus x O. aureus. Aqua. Res. 2007;38(15):1645–53.
CrossRef - Gopalraaj J., Velayudhannair K., Arockiasamy J. P., Radhakrishnan DK. The effect of dietary supplementation of proteases on growth, digestive enzymes, oxidative stress, and intestinal morphology in fishes – A review. Aquac. Int. 2023
CrossRef - Castillo S., Gatlin D. M. Dietary supplementation of exogenous carbohydrase enzymes in fish nutrition: A review. Aquaculture 2015;435:286–92.
CrossRef - Lasekan O., Hussein F. K. Classification of different pineapple varieties grown in Malaysia based on volatile fingerprinting and sensory analysis. Chem. Cent. J. 2018;12(1):140.
CrossRef - Ketnawa S., Chaiwut P., Rawdkuen S. Pineapple wastes: A potential source for bromelain extraction. Food Bioprod. Process. 2012;90(3):385–91.
CrossRef - Ketnawa S., Rawdkuen S., Chaiwut P. Two phase partitioning and collagen hydrolysis of bromelain from pineapple peel Nang Lae cultivar. Biochem. Eng. J. 2010;52(2–3):205–11.
CrossRef - Farid Hossain Md. Nutritional Value and Medicinal Benefits of Pineapple. Int. J. Food. Sci. Nutr. 2015;4(1):84.
CrossRef - Sharma S., Velayudhannair K., Arulraj J. Impact of Ananas comosus extract supplementation on the growth and biochemical profile of Cyprinus carpio fingerlings. Trends Fish. Res. 2019;8(2):69–77.
- Sharma S. A., Surveswaran S., Arulraj J., Velayudhannair K. Bromelain enhances digestibility of Spirulina-based fish feed. J. Appl. Phycol. 2021;33(2):967–77.
CrossRef - Adeoye A. A., Jaramillo-Torres A., Fox S. W., Merrifield D. L., Davies S. J. Supplementation of formulated diets for tilapia (Oreochromis niloticus) with selected exogenous enzymes: Overall performance and effects on intestinal histology and microbiota. Anim. Feed. Sci. Technol. 2016;215:133–43.
CrossRef - Yuangsoi B., Klahan R., Charoenwattanasak S., Lin S. M. Effects of supplementation of pineapple waste extract in diet of Nile tilapia (Oreochromis niloticus) on growth, feed utilization, and nitrogen excretion. J. Appl. Aquac. 2018;30(3):227–37.
CrossRef - Aravind K. V., Gokulakrishnan. Extraction, purification of bromelain from pineapple and determination of its effect on bacteria causing periodontitis. Int. J. Pharm. Sci. Res. 2015;6(12):5284–94.
- Kaur H. Analytical Chemistry. 3rd ed. Meerut: Pragati prakashan; 2015.
- Lowry O., Rosebrough N., Farr. A. L., Randall R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–75.
CrossRef - Lee Y. P., Takahashi T. An improved colorimetric determination of amino acids with the use of ninhydrin. Anal. Biochem. 1966,14(1):71–7.
CrossRef - Folch J., Lees M., Sloane Stanley G. H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226(1):497–509.
CrossRef - Ludwig T. G., Goldberg H. J. V. The Anthrone Method for the Determination of Carbohydrates in Foods and in Oral Rinsing. J. Dent. Res. 1956;35(1):90–4.
CrossRef - Rao Y. V., Chakrabarti R. Enhanced anti-proteases in Labeo rohita fed with diet containing herbal ingredients. Indian J. Clin. Biochem. 2004;19(2):132–4.
CrossRef - Citarasu T., Sivaram V., Immanuel G., Rout N., Murugan V. Influence of selected Indian immunostimulant herbs against white spot syndrome virus (WSSV) infection in black tiger shrimp, Penaeus monodon with reference to haematological, biochemical and immunological changes. Fish Shellfish Immunol. 2006;21(4):372–84.
CrossRef - Divu D., Rao K. S., Philipose K. K. Fish growth parameters and their monitoring. In: Handbook on Open Sea Cage Culture. Karwar: Central Marine Fisheries Research Institute; 2012. p. 112–7.
- Rostika R., Nurhayati A., Buwono I. D., Rizal A., Dewanti L. P., Maulana T. Papain and bromelain crude enzyme extract in commercial feed, effectiveness toward pisciculture production of striped catfish (Pangasianodon hypophthalmus) in aquaculture facility. AACL Bioflux. 2018;11:1598–604.
- Wiszniewski G., Jarmołowicz S., Hassaan M. S., Soaudy M. R., Kamaszewski M., Szudrowicz H., et al. Beneficial effects of dietary papain supplementation in juvenile sterlet (Acipenser ruthenus): Growth, intestinal topography, digestive enzymes, antioxidant response, immune response, and response to a challenge test. Aqua.c Rep. 2022;22:100923.
CrossRef - Wiszniewski G., Jarmołowicz S., Hassaan M. S., Kamaszewski M., Szudrowicz H., Terech-Majewska E., et al. Dietary effect of actinidin enzyme on growth, digestive enzymes activity, immunity, liver and intestine histology of juvenile sterlet sturgeon (Acipenser ruthenus). Aquac. Rep. 2022;25:101–96.
CrossRef - Subandiyono., Hastuti S., Nugroho R. A. Feed utilization efficiency and growth of Java barb (Puntius javanicus) fed on dietary pineapple extract. AACL bioflux. 2018;11(2).
- Choi W. M., Lam C. L., Mo WY, Wong MH. Upgrading food wastes by means of bromelain and papain to enhance growth and immunity of grass carp (Ctenopharyngodon idella). Environ. Sci. Pollut. Res. 2016, 20;23(8):7186–94.
CrossRef - Rachmawati D., Samidjan I. The Effects of Papain Enzyme Supplement in Feed on Protein Digestibility, Growth and Survival Rate in Sangkuriang Catfish (Clarias sp). Omni-Akuatika. 2018;14(2).
CrossRef - Tewari G., Ram R.N., Singh A. Effect of plant based digestive enzyme ‘papain’ on growth, survival and behavioural response of Cyprinus carpio. Int. J. Fish. Aquat. Stud. 2018;6(3):210–4.
- Hassaan M. S., Mohammady E. Y., Soaudy M. R., Elashry M. A., Moustafa M. M. A., Wassel M. A, et al. Synergistic effects of Bacillus pumilus and exogenous protease on Nile tilapia (Oreochromis niloticus) growth, gut microbes, immune response and gene expression fed plant protein diet. Anim. Feed. Sci. Technol. 2021;275:114892.
CrossRef - Herawati T., Yustiati A., Nurhayati A., Mustikawati R. Proximate composition of several fish from Jatigede Reservoir in Sumedang district, West Java. IOP Conf Ser Earth Environ Sci. 2018;137:012055.
CrossRef - Li P., Mai K., Trushenski J., Wu G. New developments in fish amino acid nutrition: towards functional and environmentally oriented aquafeeds. Amino Acids. 2009;37(1):43–53.
CrossRef - Gómez-Limia L, Franco I, Blanco T, Martínez S. Changes in amino acids content in muscle of European eel (Anguilla anguilla) in relation to body size. Int. J. Agric. Biol. 2019;13(2):38–41.
- Krogdahl A., Hemre G. I., Mommsen T. P. Carbohydrates in fish nutrition: digestion and absorption in postlarval stages. Aquac. Nutr. 2005;11(2):103–22.
CrossRef - Prabu E., Felix S., Felix N., Ahilan B., Ruby P. An overview on significance of fish nutrition in aquaculture industry. Int. J. Fish. Aquat. Stud. 2017;5(6):349–55.
- Kuebutornye F. K. A., Roy K., Folorunso E. A., Mraz J. Plant-based feed additives in Cyprinus carpio aquaculture. Rev. Aquac. 2023.
CrossRef - Hassaan M. S., El-Sayed A. I. M., Soltan M. A., Iraqi M. M., Goda A. M., Davies S. J., et al. Partial dietary fish meal replacement with cotton seed meal and supplementation with exogenous protease alters growth, feed performance, hematological indices and associated gene expression markers (GH, IGF-I) for Nile tilapia, Oreochromis niloticus. Aquaculture 2019; 503:282–92.
CrossRef - Saleh E. S. E., Tawfeek S. S., Abdel-Fadeel A. A. A., Abdel-Daim A. S. A., Abdel-Razik A. R. H., Youssef I. M. I. Effect of dietary protease supplementation on growth performance, water quality, blood parameters and intestinal morphology of Nile tilapia (Oreochromis niloticus). J. Anim. Physiol. Anim. Nutr. (Berl). 2022;106(2):419–28.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





