How to Cite | Publication History | PlumX Article Matrix
Smita P. Kakad* , Yash R. Bharati
, Yash R. Bharati , Sanjay J. Kshirsagar
, Sanjay J. Kshirsagar  , Neelam Dashputre
, Neelam Dashputre , Anjali Tajanpure
, Anjali Tajanpure , Rani S. Kankate
, Rani S. Kankate , Pratibha Maurya
, Pratibha Maurya and Shalaka Dhikale
and Shalaka Dhikale
Department of Pharmaceutics, MET Institute of Pharmacy, Savitribai Phule Pune University, Adgaon, Nashik, Maharashtra, India
Corresponding Author E-mail: smitadarade87@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3207
ABSTRACT: Background: This research was aimed with the development of antipsychotic drug delivery for olfactory administration which could deliver drug to the brain. Amisulpride is a psychoactive drug that belongs to the benzamide derivatives class. It enhances dopaminergic neurotransmission by inhibiting presynaptic dopamine D2/D3 auto receptors selectively at lower dosages. Method: The nanosuspension was prepared by media milling technique for nose to brain delivery. The nose to brain delivery developed an effective route to bypass the BBB and deliver the drug to the brain. Factorial design was used for the designing and optimizing formulation based on various process and formulation factors. The optimized batch further analyzed to determine particle size, PDI, zeta potential, and drug content. With appropriate selection of process parameters like speed and bead amount. The media milling method is one of the effective methodology to reduce particle size and with the help of stabilizers nanoparticles could be stabilised. Result: The average particle size range of nanosuspension batch was observed 100-150 nm with a polydispersity index of 0.0927, Zeta potential +39.14 mV and drug content 88.12 ± 2 %. Conclusion: Intranasal administration is a promising alternative for bypassing the blood-brain barrier, reducing the adverse effects, and lowering the doses.
KEYWORDS: Amisulpride; Antipsychotic; Brain Targeting; Media Mill; Nanosuspension
Download this article as:| Copy the following to cite this article: Kakad S. P, Bharati Y. R, Kshirsagar S. J, Dashputre N, Tajanpure A, Kankate R. S, Maurya P, Dhikale S. Fabrication of Amisulpride Nanosuspension for Nose to Brain Delivery in the Potential Antipsychotic Treatment. Biotech Res Asia 2024;21(1). |
| Copy the following to cite this URL: Kakad S. P, Bharati Y. R, Kshirsagar S. J, Dashputre N, Tajanpure A, Kankate R. S, Maurya P, Dhikale S. Fabrication of Amisulpride Nanosuspension for Nose to Brain Delivery in the Potential Antipsychotic Treatment. Biotech Res Asia 2024;21(1). Available from: https://bit.ly/3wyfXGR |
Introduction
At present majority of the antipsychotics are available to use with oral and injectable route. There is a requirement of alternative route to improve patient compliance and bioavalability1.The need for option in contrast to oral route is obvious in cases where a patient is not in treatment receiving mode, not able to swallow or suffer from gastrointestinal upset. Many antipsychotic drugs are inappropriate with oral route due to less efficacy as a result of gastrointestinal metabolism, hepatic first-pass metabolism, and enzymatic breakdown in the gastrointestinal system. Besides, injectable formulations are not practically possible for all drugs because of aqueous solubility and unfavourable physical properties 2. Psychosis can be characterized with positive and negative signs. Positive appearances address over-production or distortion of normal function (for example hallucination, delusion, and disturbing speed). Negative appearances address absences of ordinary capacities, for example, smoothed effect a violation and diminished speech (i.e., alogia)3.
Amisulpride exhibit antipsychotic activities by selective binding to dopamine D2 and D3 receptors in the limbic system. It is used in the treatment of psychosis, neurotics, productive schizophrenia dysthymia. Regardless, amisulpride (AMP) is a poorly water-soluble drug, so solvency is the principal constraint for oral bioavailability. Amisulpride is a drug that has been approved for the treatment of both acute and chronic schizophrenia symptoms that are either positive or potentially negative4. Advantages and disadvantage associated with nasal delivery are listed in (Table.1) 5, 6 & 7
Nasal route allows the drug to quickly absorbed without the biotransformation.5,6 Olfactory nerve and trigeminal nerve aids direct access of drugs to the brain by circumventing the blood brain barrier (BBB).8 The veins providing blood to the brain have efflux transporter located at endothelial cells.9
Nose to the brain drug delivery is by direct and indirect route. Direct route is via olfactory way bypassing BBB and indirect transport via blood brain barrier.10 Several formulation strategies are available to deliver drug via nasal cavity bypassing BBB.10,11 Devices like “Via Nose electronic atomizer” and “Opti Nose bi-directional delivery system” are available for such delivery11. Nasal retention of solution is a prime requisite for which fine spray is alternative12. Other objectives are to achieve good permeation, and bypass efflux transport.13, 14 Nasal formulation includes solubilizers, penetration enhancers, mucoadhesive compounds or viscosity enhancing agent, or thermo responsive substances to improve the drug residence time. Some approaches where drug is encapsulated in nano size carriers that, facilitate brain transport compared with drug dispersions.15,16,17
Around 40% of the drug molecules are unused by the pharmaceutical industry due to insufficient aqueous solubility of active pharmaceutical ingredients.18,19 Some molecules have better availability in systemic circulation but have lesser concentration at central nervous sytem.20 Coupling the benefits of nanocapsulation enhances drug loading, improved transport, minimize drug requirement with these benefits intranasal delivery empowers the testing of recently rejected APIs.21 Specially designed devices are available for nasal drug delivery for example mucosal atomizer device. In this device when a medicine is sprayed into the nose, it is rapidly absorbed through the inner surface of the nose, without delay, into the blood.22 In Opti nose bi-directional delivery system the revolutionary mechanism of motion is used. Opti nose design allow broad, steady drug deep in the nasal passages, leveraging the mucosal surfaces as a possible goal for the remedy of local and systemic effects.23
Method
Chemicals and reagent used
Amisulpride was gifted from Sanofi India Limited Goa, glycerine (Research Lab and Fine Chem. Industries), Poloxamer 407(Glenmark Pharmaceuticals Ltd R and D centre, Sinner), Sodium hydroxide (Thomas Baker, Mumbai), potassium dihydrogen phosphate (SDFCL, Mumbai). All ingredients and solvents utilized were of analytical grade.
Preformulation studies
The received sample of Amisulpride was subjected to a preformulation study.24 For meting point determination small amount of drug was placed in the capillary tube which was previously sealed on one side, tied with a thermometer which was placed in a Thiele tube in which liquid paraffin was added and Thiele tube arm heated with Bunsen burner. The sample was observed and the temperature range between which sample starts melting was noted as melting of the sample.
UV visible spectrometric method for analysis
Amisulpride stock solution was prepared in methanol (100μg/ml) and phosphate buffer solution (PBS 6.5). Dilutions were prepared with solvents within 2-10 μg/ml. Analysis was done by in the range 200 to 400 nm by using a UV visible spectrometer (V630Jasco, India). 24 This calibration graph was used for the estimation of drug during in vitro drug release studies and saturation solubility studies.25
FTIR analysis
FTIR spectrophotometer (Shimadzu Co., Japan) was used to record the spectrum of formulation components. The drug: KBr, (1:9) was used for analysis. FTIR spectrum helps to elucidate structural interpretation and functional group interpretation as well as interactions between components.26,28
Formulation of nanosuspension
Nanosuspension was formulated by using the media milling. Glycerine was dissolve in water. Poloxamer 407 was added to above glycerine and water solution. Add milling media (Zirconium bead) with specified quantity in vial and stirred them using magnetic stirrer. Drug was dispersed in above solution by slow addition.This mixture was stirred using magnetic stirrer and stirred for 3 hour at 1500-2000 rpm.27,28
Trial batches using media milling method
Trial batches prepared with media milling are mentioned in table 2. Formulation variables were polymer and surfactant. Process variables were time and speed of magnetic stirrer.
Table 1: Trial Batches using media milling method
| Batch
No. |
Drug
|
Polox. 188
|
Polox. 407
|
HPMC 5Cps | Glycerol | Water q.s | Beads | Speed |
| % w/v | gm | RPM | ||||||
| T1 | 0.1 | – | 0.1 | 0.1 | – | 100 | 10 | 1000 |
| T2 | 0.1 | – | 0.05 | – | – | 100 | 10 | 1000 |
| T3 | 0.1 | – | 0.1 | – | 0.1 | 100 | 12 | 1000 |
| T4 | 0.1 | 0.05 | 0.05 | – | – | 100 | 10 | 1000 |
| T5 | 1 | – | 0.1 | – | 0.1 | 100 | 12 | 1000 |
Optimization of formula by 3 factors and 2 levels
The ideal nanosuspension composition was established using the experiment’s design. The nanosuspension was assessed for Zeta potential, particle size distribution, and particle size. In the formulation, poloxamer 407 was utilized as a stabilizer. Effect on particle size was noted as a dependant variable. For experimnetal optimization, Design Expert®12 was used. 3 factors 2 levels factorial design was used to analysed the effect. The factor selected were Poloxamer 407, bead (gm), and milling speed (RPM) with the level of -1, +1 (low and high). Poloxamer 407 concentration 0.05 and 0.1%, bead with 10 and 12 g and speed with 1000 and 1200 RPM. Batch composition is mentioned in table 3. ANOVA was applied to analyze statistical significance at p<0.05.29,30
Table 2: Composition of nanosuspension by 23 level factorial designs
| Batch | Amisulpride
|
Glycerol
|
Polox. 407
|
Bead
|
Speed
|
| %w/v | gm | RPM | |||
| F1 | 1 | 0.1 | 0.5 | 10 | 1200 |
| F2 | 1 | 0.1 | 1 | 10 | 1200 |
| F3 | 1 | 0.1 | 0.5 | 12 | 1200 |
| F4 | 1 | 0.1 | 1 | 12 | 1200 |
| F5 | 1 | 0.1 | 0.5 | 10 | 1000 |
| F6 | 1 | 0.1 | 1 | 10 | 1000 |
| F7 | 1 | 0.1 | 0.5 | 12 | 1000 |
| F8 | 1 | 0.1 | 1 | 12 | 1000 |
Characterization of Nanosuspension
Determination of pH
A pH probe was then inserted into approximately 20 ml formulation to measure its pH, reading was displayed on the digital pH meter from Systronics (India).31
Determination of polydispersity index and particle size
NANOPHOX (SYMPATEC Germany) instrument was used to measure polydispersity and average particle size of the nanosuspension. A laser beam is used to illuminate the sample, and as it scatters, the light’s intensity varies at a rate that depends on the particle’s size at 25°C using a small volume disposable polystyrene cuvette.28,31 this method involves principal of dynamic scattering of light.
Determination of zeta potential
Zeta potential was measured using Beckman Coulter equipment. The surface charge and potential was measured.29
Characterization by DSC
DSC guides about the amount of heat absorbed or emitted by the sample, and to assess any polymorphic transformations that could occur during processing steps. The shifting of melting temperature can be detected with the use of DSC. DSC measurement of pure drug and the optimized formulation was performed. The samples were dried and 2-5mg sample was precisely weighed and placed for further analysis.32
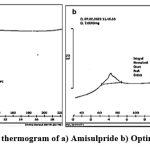 |
Figure 1: DSC thermogram of a) Amisulpride b) Optimized batch |
Determination of drug content of nanosuspension
From the nanosuspension batch 1 ml was diluted with methanol filter with Whatman filter paper (0.45 μm). The material was examined using a UV spectrophotometer at a maximum 226nm wavelength. Equation (1) helps to determine the total drug content (TDC): 33

In vitro Diffusion study
Franz diffusion cells with a 16 ml volume capacity was used. A dialysis membrane (MWCO 12,000g/mol.) used for as media to asses drug transport with the nanosuspension equivalent to 5mg of Amilsulpride. Dialysis membrane was positioned between donor compartment and receptor compartments. The magnetic stirrer was kept at a temperature of 37°C and swirled constantly at 100 RPM. PBS 6.5 was added to the receiver compartment, and samples were taken out at regular intervals and replaced with new medium in the same volume. Drug release was measured at 226 nm.34,35
Ex-Vivo Diffusion study
For the optimized batch (F8), diffusion release was performed on goat nasal mucosa. The mucosal membrane functions as a permeation membrane for the diffusion of drugs.36 The diffusion medium, PBS 6.5, was swirled continuously at 100 RPM on a magnetic stirrer kept at 37°C. At regular intervals, the sample was taken out and the same volume was added to a new medium. Using a UV spectrophotometer, the material was evaluated at 226 nm. 34.
Histopathological study
Nasal irritation or cell wall damage after intranasal administration of product could be check by histopathological analysis on excised goat nasal mucosa. From the local slaughterhouse excised goat nasal mucosa was obtained and placed in PBS 6.4. It was washed with PBS and divided into three symmetrical parts. Using 70% isopropyl alcohol as a positive control on the first, PBS 6.4 as a negative control on the second, and the formulation on the third, the first was treated. All samples were treated for 1 hour with phosphate buffer solution, followed by 24 hours of immediate storage in 10% formalin solution. In order to dehydrate the material, replace the formalin solution with 70% ethanol after 24 hours and placed at 4°C. Using a microtome, sliced dehydrated sections with thickness of 5mm and placed in agar and paraffin. Staining was done with haematoxylin dye and eosin dye mixture further observations under microscope (TUCSENUSB2.0H) for any significant change.37,38
Stability study
The stability study was conducted in accordance with ICH standards. The final mixture was tested for stability, and it was sealed in borosilicate glass vials, which were then held at 40°C/2°C/75%RH5% for six months.38
Results
The drug was observed for colour, odour, and melting point. In melting point, physical changes such as solid to the liquid form of pure drug/API were observed and compared with their standard reference values. By comparing practical and reference values it was observed that the given sample of Amisulpride was pure. The reported λ max of Amisulpride in methanol, distilled water and PBS 6.5 was 226 nm. The calibration curve is found to be linear and the coefficient of regression is in the acceptable range. All the calibration plots followed Lamberts Beer’s law. 24,26
Solubility analysis
Amisulpride poorly dissolves in PBS 6.5. Solubility results are mentioned in table 4. Rise in the drug’s solubility is a result of nanosizing. In water and PBS 6.5, solubility of drug increases by 10-15 and 9-10-fold, respectively. This occurs as a result of media milling techniques that use various polymers and surfactants to reduce particle size. Surfactants aid in lowering the drug’s hydrophobicity39. The increase in surface area caused by the reduction in particle size enhances the particle surface exposure to the medium.
Table 3: Solubility study of Amisulpride
| Sr. No | Solvents | Amisulpride Solubility (mg/ml) | Solubility of Nanosuspension Formulation (Optimized batch) Solubility (mg/mL) |
| 1. | Water | 0.0291 ±0.15 | 0.312 ±0.25 |
| 2. | PBS 6.5 | 0.0379±0.19 | 0.355±0.27 |
FTIR analysis
Amisulpride’s melting point was determined to be 129.69 °C. The spectrum of the medicine reveals a sharp endothermic peak and a fast decline in heat flux, indicating that the drug has melted. Amisulpride’s real melting point is between 128 and 130 °C. We conclude that amisulpride is in pure form since the spectrum indicates that melting begins around 126.01 °C.Figure 2 depicts the well-characterized transitions in the FTIR spectrum of the improved formulation at 149.2° C. Amisulpride’s real melting point is between 128 and 130 °C. Amisulpride’s peak shift to an exothermic peak is the result of a change in physical state brought on by the media milling process, which aids in particle size reduction. We can infer that the pure drug’s amorphous character was transformed into a crystalline one with the application of thermal energy.32
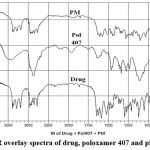 |
Figure 2: FTIR overlay spectra of drug, poloxamer 407 and physical mixtur |
Optimization by 3 factors and 2 level factorial designs
The study employed a 3 component and 2-level factorial design. All 8 probable pairings were the subject of experimental tests. The components shown in figure 3 were the speed (RPM) (X1), bead (X2), and amount of stabilizer concentration (X3). Utilizing optimization tools like Design Expert 12, the statistical optimization procedure was carried out. Design summary by factorial design is mentioned in table 5. Final equation for particle size showing coded factor is equation 2.
Table 4: Experimental design summary
| Factor | Units | Actual | Coded | ||
| Level | – | Low | High | Low | High |
| A- Speed | RPM | 1200 | 1500 | -1 | +1 |
| B- Bead | Gm | 10 | 12 | -1 | +1 |
| C- Poloxamer 407 | % | 0.05 | 0.1 | -1 | +1 |
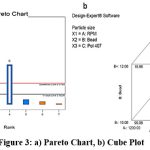 |
Figure 3: a) Pareto Chart, b) Cube Plot. |
Particle Size = +77.19+40.48*A-19.53*B-22.30*C-2.36*AB +0.41*AC+20.53*BC+1.35*ABC———-(2)
The biggest impact on the particle size of nanosuspension is caused by speed (Factor A) and the interaction between (Factors B and C), according to the specified criteria. The particle size reduces as we increase speed, which has a negative impact on the particle size. Also, with the decrease in the amount of Poloxamer 407, there is a reduction in particle size observed. Predicted and experimental results of particle size in nanosuspension were given in table 6.
Table 5: Predicted Results and experimental results
| Parameter | Desired value | Predicted response | Experimental response | Residual error |
| Particle size (nm) | <250 | 128.672 | 130.001 | 1.032% |
Interaction occurs when the response is distinctive depending upon the settings of two variables. It indicates that the effect of one factor relies upon the level of the other. From the below figure of interaction between factors AB and AC, it is observed that two planes are parallel to one another which means there is no interaction observed between factors but with the factor BC observe that there is the interaction of factors B and C the change is in the positive direction. The effect of change in particle size is dependent on the level of Bead and Poloxamer 407. (fig. 4).
 |
Figure 4: Interaction plot factors a) Factor AB Factor BC, b) Factor BC Factor AC |
The effect of RPM, Bead and particle size was studied as shown in the 3D plot and counterplot. From the counterplot and 3D plot, we observed that at a low level of RPM and Bead there is more particle size. At the point when the level is increased, there is a reduction in particle size observed. So, RPM, Bead shows the opposite effect on particle size. (Fig. 5).
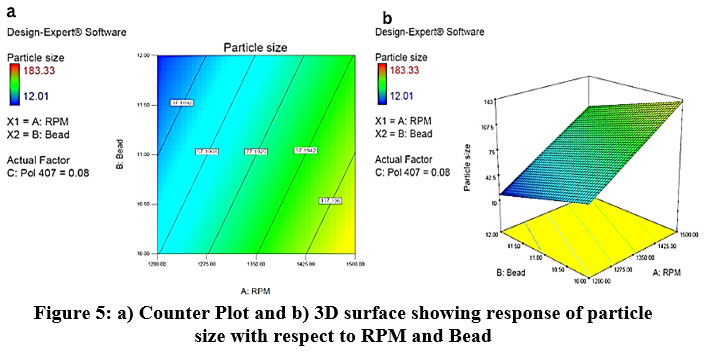 |
Figure 5: a) Counter Plot and b) 3D surface showing response of particle size with respect to RPM and Bead. |
As per statistical data, 100 solutions were obtained from optimization software. Formulation F8 showed lesser particle size and optimum zeta potential.(fig.8) The experimental results were compared with the predicted response and residual error was calculated. Lower percentage of error indicates that results given by the software are appropriate in accordance with the predictions39.
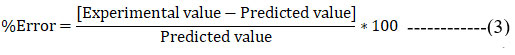
Evaluation studies of formulation
PDI and Particle size
Particle size has been reduced using the media milling technique. By altering the formulation and process parameters, particle size can be reduced. The ideal size range for drugs being delivered to the brain is <300 nm 41. Batch F8 displays a lesser size 99.15 nm with PDI 0.0927, speed of 1500 RPM, 0.1% poloxamer 407, and 12 gm of the bead. The uniformity in size increases as the polydispersity index decreases.
Value of PDI 0.0927 narrow size distribution is confirmed. A crucial parameter that can be used to evaluate the stability and biopharmaceutical properties of nanosuspension is particle size distribution. The small size of gives a larger surface area for absorption of drug via biological membrane. 29,31 Figure 6 showed PDI, Particle size, drug content, pH, in vitro % drug release of optimize batches of nanosuspension is mentioned in table.7 Intranasal pH ranges are between 4.5 and 6.5. If formulation pH is within same range, it will prevent irritation after application.42 Batch B8 showed pH 6.1.
 |
Figure 6: PDI, Particle Size of F8 Batch |
Zeta Potential of optimized batch
 |
Figure 7: Zeta potential of optimized batch |
Zeta potential of disperse particles should be more than 30 mV for better stability; however, the range should be 20mV to 30mV. The Zeta potential of F8 batch was found to be +39.14 mV. It is important parameter to measure long-term stability of dispersions. To create a stable nanosuspension, stabilizer, surfactant, and polymers were combined to reduce particle aggregation. (fig.7).
Drug content
Drug content of nanosuspension is given in (Table.7). It is found within 86-95%. F8 batch shows 91.96 %. Drug content is observed less because of human error during processing, milling and transfer of batches.42, 43, 44&45
Table 6: Characterization of F8 batch
| Formulation | Particle size
(nm) |
Polydispersity index
|
pH | Drug content (%) | In vitro% release |
| F1 | 95.77 | 0.0930 | 4.9± 0.3 | 90.54± 3.048 | 76.20± 0.669 |
| F2 | 12.01 | 0.6744 | 5.6± 0.4 | 86.21± 1.256 | 7.150 ± 1.243 |
| F3 | 23.08 | 0.3119 | 5.8± 0.2 | 93.29± 1.990 | 7.150 ± 1.243 |
| F4 | 16.01 | 0.3185 | 6.6± 0.1 | 88.76± 3.052 | 8.207± 1.243 |
| F5 | 183.88 | 0.0943 | 5.7± 0.3 | 92.15± 2.149 | 8.079± 0.642 |
| F6 | 95.78 | 0.0923 | 5.3± 0.2 | 95.31± 1.756 | 8.465± 1.536 |
| F7 | 93.79 | 0.0919 | 5.7± 0.8 | 89.10± 1.990 | 7.540± 0.642 |
| F8 | 99.15 | 0.0927 | 6.1± 0.2 | 91.96± 2.149 | 8.812± 0.954 |
In-vitro diffusion study
The formulation batch F8 with a smaller particle size exhibits higher diffusion, according to the diffusion analysis. In 60 minutes, a nanosuspension with particles that are 99.15 nm in size reveals an 88.12% drug release. Results are displayed in table 7. The drug’s crystalline state is changed to amorphous by media milling (zirconium beads), which increases solubility by reducing particle size and increasing surface area. This also aids in improving drug diffusion from the membrane and improving bioavailability. Four models -Zero, first order, Higuchi, and Korsmeyer-Peppas Model) were fitted with the drug release kinetic data. The Higuchi model yielded the highest R2 value.32,42&44
Ex-Vivo study
Excised goat nasal mucosa was subjected for an ex-vivo study. Formulation F8 was used in this study.The nasal membrane was permeable to 87.68% of drug releases. The medication diffuses more quickly across nasal mucosa, and its overall percentage diffusion is lower than its diffusion across the dialysis membrane. It’s possible that the goat nasal mucosa’s closely bonded epithelial cells account for the reduced permeation. Given that mucociliary clearance occurs quickly in vivo, high mucosal permeability is desirable. takes the medication out of the nasal mucosa. The nasal membrane will be more permeable the smaller the particle size. This may enable the transcellular delivery of nanosuspension to the brain from the olfactory and trigeminal nerves.41 Results are mentioned in table 8.
Table 7: Ex-Vivo permeation study of F8 batch
| Time (min) | Drug release (%) |
| 5 | 35.75327± 1.048 |
| 10 | 42.15673± 1.667 |
| 15 | 50.95204± 2.934 |
| 30 | 63.87367± 2.905 |
| 45 | 79.87833±1.463 |
| 60 | 87.68449± 1.193 |
Histopathological study
In order to make sure that the nasal mucosa is not significantly harmed by the improved nanosuspension formulation and that the epithelial cells are arranged sequentially, a nasal histopathology observed. Figure 8.A, positive control sample in 70% IPA isopropyl alcohol exhibits damaging effect to epithelial cells, causing them to get detached from the membrane and distort their normal order in comparison to the negative control sample figure B. After receiving treatment with nanosuspension, epithelial cells showed no discernible signs of deterioration. Showing no harm to nasal cell linings shown in figure C.38,42
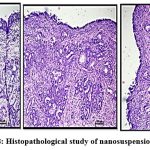 |
Figure 8: Histopathological study of nanosuspension (A,B,C) |
Stability study
For three months, stability chamber conditions were maintained at 75% ± 5% relative humidity and 40°C ± 2°C. Drug content and drug release were examined. Formulation batches were examined after three months results shown in table 8. From the investigation, it did not show significant change in the response after storage.44
Table 8: Accelerated stability study of Optimized nanosuspension (F8)
| Temperature and humidity | Parameter | Months (3) |
| 40ᴼC±2ᴼC;75% ± 5%RH | Drug content (%)
(F8) |
89.73 ± 0.940 |
| Drug release (%)
(F8) |
88.03 ± 0.156 | |
| Room temperature (25 ᴼC) | Drug content (%)
(F8) |
90.17± 0.093 |
| Drug release (%)
(F8) |
87.83± 0.068 |
Discussion
Nanosuspension solved the poor bioavailability problem of hydrophobic drugs. There are several formulation approaches to resolving the problem related to low solubility and low bioavailability. Nanosuspension enhanced solubility and bioavailability. Production methods such as media milling technique and high-pressure homogenization could utilized for scaleup. Patented technologies and devising techniques allowed drug to cross blood-brain barrier and reach CNS. Directly targeting the brain using nasal route shows greater potential, with less systematic side effects. Nasal route successfully delivers potent drugs to CNS.
Process parameter determines the success of the media milling process in terms of reduction in particle size, the study of solubility of Amisulpride results rise in solubility after nanosizing. Ratio of stabilizers is also equally important for stabilizing the particles by minimizing agglomeration and flocculation. The media milling method was chosen as the technique of choice. The speed of the stirrer leads to a reduction in particle size, which leads to an increase in surface area and finally an increase in solubility and drug release. The optimized batch was found to be F8 by design expert software which has minimum particle size, polydispersity index, optimize Zeta potential, and improved drug release. The particle of the F8 batch was 99.15 nm, PDI was 0.0927, zeta potential was +39.14 mV and drug release 88.12 %. In the FTIR study it is observe that all the components that are being used in the formulation which are drug and excipients are compatible with each other and shows no interaction either physical and chemical between them. The histopathological study clarified that the formulation is not damaging the nasal mucosa membrane so it is considered as a safe formulation. Stability study of formulation at different temperature and humidity conditions was studied to evaluate drug and excipient interaction and effect on drug release and formulation was found to be stable as drug content of the formulation did not show significant change in the response after storage.
Conclusion
In the present study, physically stable formulation of Amisulpride nanosuspension was formulated. Intranasal delivery of Amisulpride nanosuspension is assumed to be promising approach in treatment of psychosis. Factorial design was used for optimizing the formulation. Appropriate selection of process parameters (speed and bead amount) we can conclude that the media milling method is an effective methodology to reduce the particle size with the help of a stabilizer. Rapid absorption produces a plasma profile for a lipophilic medication with a 1000 Dalton molecular weight that is comparable to intravenous delivery. Enhanced bioavailability contrast with oral administration because of avoidance hepatic or gastrointestinal metabolism.
Acknowledgement
I extend my sincere gratitude to my guide whose work has contributed to my understanding of these research. I also wish to thank my friends and peers for their constructive feedback and support throughout the writing process.
Author Contribution
All authors contributed in this work.
Conflict of interest
The authors report no conflicts of interest.
Funding Sources
This study was self-funded.
Ethical statement
Not Applicable
Data availability
Not Applicable
References
- Kaminsky BM, Bostwick JR, Guthrie SK. Alternate routes of administration of antidepressant and antipsychotic medications. Annals of Pharmacotherapy. 2015 Jul;49(7):808-17.
CrossRef - Katare YK, Piazza JE, Bhandari J, Daya RP, Akilan K, Simpson MJ, Hoare T, Mishra RK. Intranasal delivery of antipsychotic drugs. Schizophrenia research. 2017 Jun 1;184:2-13.
CrossRef - Cohen JA, Issues TW, AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with posttraumatic stress disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2010 Apr 1;49(4):414-30.
CrossRef - Abd Elbary A, Ali AA, Aboud HM. Enhanced dissolution of meloxicam from orodispersible tablets prepared by different methods. Bulletin of Faculty of Pharmacy, Cairo University. 2012 Dec 1;50(2):89-97.
CrossRef - Bitter C, Suter-Zimmermann K, Surber C. Nasal drug delivery in humans. Topical Applications and the Mucosa. 2011;40:20-35.
CrossRef - Grassin-Delyle S, Buenestado A, Naline E, Faisy C, Blouquit-Laye S, Couderc LJ, Le Guen M, Fischler M, Devillier P. Intranasal drug delivery: an efficient and non-invasive route for systemic administration: focus on opioids. Pharmacology & therapeutics. 2012 Jun 1;134(3):366-79.
CrossRef - Mathias NR, Hussain MA. Non-invasive systemic drug delivery: developability considerations for alternate routes of administration. Journal of pharmaceutical sciences. 2010 Jan 1;99(1):1-20.
CrossRef - Kakad S, Kshirsagar S. Nose to brain delivery of Efavirenz nanosuspension for effective neuro AIDS therapy: in-vitro, in-vivo and pharmacokinetic assessment. Heliyon. 2021 Nov 1;7(11):e08368.
CrossRef - Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Advanced drug delivery reviews. 2012 May 15;64(7):614-28.
CrossRef - Mulam TR, Kshirsagar SJ, Kakad SP. Formulation and optimization of ritonavir nasal nanosuspension for brain targeting. Indian Drugs. 2021 Apr;58(4):28-41.
CrossRef - Djupesland PG, Skretting A. Nasal deposition and clearance in man: comparison of a bidirectional powder device and a traditional liquid spray Journal of aerosol medicine and pulmonary drug delivery. 2012 Oct 1;25(5):280-9.
CrossRef - Piazza JE, Zhu C, Ravi Selvaganapathy P, R Hoare T, Jain SB, Hossain F, Mishra RK. A Novel Intranasal Spray Device for the Administration of Nanoparticles to Rodents. Journal of Medical Devices. 2015 Dec 1;9(4).
CrossRef - Costantino HR, Illum L, Brandt G, Johnson PH, Quay SC. Intranasal delivery: physicochemical and therapeutic aspects. International journal of pharmaceutics. 2007 Jun 7;337(1-2):1-24.
CrossRef - Kakad SP, Kshirsagar SJ. Neuro-AIDS: current status and challenges to antiretroviral drug therapy (ART) for its treatment. Current Drug Therapy. 2020 Oct 1;15(5):469-81.
CrossRef - Kozlovskaya L, Abou-Kaoud M, Stepensky D. Quantitative analysis of drug delivery to the brain via nasal route. Journal of controlled release. 2014 Sep 10;189:133-40.
CrossRef - Zhang QZ, Zha LS, Zhang Y, Jiang WM, Lu W, Shi ZQ, Jiang XG, Fu SK. The brain targeting efficiency following nasally applied MPEG-PLA nanoparticles in rats. Journal of drug targeting. 2006 Jan 1;14(5):281-90.
CrossRef - Qian S, Wong YC, Zuo Z. Development, characterization and application of in situ gel systems for intranasal delivery of tacrine. International journal of pharmaceutics. 2014 Jul 1;468(1-2):272-82.
CrossRef - Svenson S, Chauhan AS. Dendrimers for enhanced drug solubilization.
- Alavijeh MS, Chishty M, Qaiser MZ, Palmer AM. Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. NeuroRx. 2005 Oct;2(4):554-71.
- Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005 Jan;2(1):3-14.
CrossRef - Katare YK, Daya RP, Sookram Gray C, Luckham RE, Bhandari J, Chauhan AS, Mishra RK. Brain targeting of a water insoluble antipsychotic drug haloperidol via the intranasal route using PAMAM dendrimer. Molecular pharmaceutics. 2015 Sep 8;12(9):3380-8.
CrossRef - Maaz A, Blagbrough IS, De Bank PA. In Vitro Evaluation of Nasal Aerosol Depositions: An Insight for Direct Nose to Brain Drug Delivery. Pharmaceutics. 2021 Jul 14;13(7):1079.
CrossRef - Djupesland PG, Messina JC, Mahmoud RA. Breath powered nasal delivery: a new route to rapid headache relief. Headache: The Journal of Head and Face Pain. 2013 Sep;53:72-84.
CrossRef - Pharmacopoeia I. Ministry of Health and Family Welfare, Indian Pharmacopoeia Commision, Ghaziabad. Vol. III. 2014:2336.
- Baka E, Comer JE, Takács-Novák K. Study of equilibrium solubility measurement by saturation shake-flask method using hydrochlorothiazide as model compound. Journal of pharmaceutical and biomedical analysis. 2008 Jan 22;46(2):335-41.
CrossRef - Pavia DL, Lampman GM, Kriz GS, Vyvyan JA. Introduction to spectroscopy. Cengage learning; 2014.
- Lakshmi P, Kumar GA. Nanosuspension technology: A review. Int J Pharm Pharm Sci. 2010 Aug;2(4):35-40.
- Arunkumar N, Deecaraman M, Rani C. Nanosuspension technology and its applications in drug delivery. Asian Journal of Pharmaceutics (AJP). 2009;3(3).
CrossRef - Gora S, Mustafa G, Sahni JK, Ali J, Baboota S. Nanosizing of valsartan by high pressure homogenization to produce dissolution enhanced nanosuspension: pharmacokinetics and pharmacodyanamic study. Drug Delivery. 2016 Mar 23;23(3):930-40.
CrossRef - Karakucuk A, Celebi N, Teksin ZS. Preparation of ritonavir nanosuspensions by microfluidization using polymeric stabilizers: I. A Design of Experiment approach. European Journal of Pharmaceutical Sciences. 2016 Dec 1;95:111-21.
CrossRef - Sutradhar KB, Khatun S, Luna IP. Increasing possibilities of nanosuspension. Journal of nanotechnology. 2013 Jan 1;2013.
CrossRef - LaFountaine JS, Jermain SV, Prasad LK, Brough C, Miller DA, Lubda D, McGinity JW, Williams III RO. Enabling thermal processing of ritonavir–polyvinyl alcohol amorphous solid dispersions by KinetiSol® dispersing. European Journal of Pharmaceutics and Biopharmaceutics. 2016 Apr 1;101:72-81.
CrossRef - Zhang H, Zhao Y. Preparation, characterization and evaluation of tea polyphenol–Zn complex loaded β-chitosan nanoparticles. Food Hydrocolloids. 2015 Jun 1;48:260-73.
CrossRef - Chorny M, Fishbein I, Danenberg HD, Golomb G. Lipophilic drug loaded nanospheres prepared by nanoprecipitation: effect of formulation variables on size, drug recovery and release kinetics. Journal of controlled release. 2002 Oct 30;83(3):389-400.
CrossRef - Kulkarni AD, Vanjari YH, Sancheti KH, Belgamwar VS, Surana SJ, Pardeshi CV. Nanotechnology-mediated nose to brain drug delivery for Parkinson’s disease: a mini review. Journal of drug targeting. 2015 Oct 21;23(9):775-88.
CrossRef - Wheatley MA, Dent J, Wheeldon EB, Smith PL. Nasal drug delivery: an in vitro characterization of transepithelial electrical properties and fluxes in the presence or absence of enhancers. Journal of controlled release. 1988 Dec 1;8(2):167-77.
CrossRef - Erdő F, Bors LA, Farkas D, Bajza Á, Gizurarson S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain research bulletin. 2018 Oct 1;143:155-70.
CrossRef - Khan K, Aqil M, Imam SS, Ahad A, Moolakkadath T, Sultana Y, Mujeeb M. Ursolic acid loaded intra nasal nano lipid vesicles for brain tumour: Formulation, optimization, in-vivo brain/plasma distribution study and histopathological assessment. Biomedicine & pharmacotherapy. 2018 Oct 1;106:1578-85.
CrossRef - Mahajan HS, Mahajan MS, Nerkar PP, Agrawal A. Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Drug delivery. 2014 Mar 1;21(2):148-54.
CrossRef - Zhou YZ, Alany RG, Chuang V, Wen J. Optimization of PLGA nanoparticles formulation containing L-DOPA by applying the central composite design. Drug development and industrial pharmacy. 2013 Feb 1;39(2):321-30.
CrossRef - Pardeshi CV, Belgamwar VS. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood–brain barrier: an excellent platform for brain targeting. Expert opinion on drug delivery. 2013 Jul 1;10(7):957-72.
CrossRef - Ohwaki T, Ando H, Watanabe S, Miyake Y. Effects of dose, pH, and osmolarity on nasal absorption of secretin in rats. Journal of pharmaceutical sciences. 1985 May 1;74(5):550-2.
CrossRef - Prabhakar K, Afzal SM, Surender G, Kishan V. Tween 80 containing lipid nanoemulsions for delivery of indinavir to brain. Acta Pharmaceutica Sinica B. 2013 Sep 1;3(5):345-53.
CrossRef - Kakad SP, Gangurde TD, Kshirsagar SJ, Mundhe VG. Nose to brain delivery of nanosuspensions with first line antiviral agents is alternative treatment option to Neuro-AIDS treatment. Heliyon. 2022 Jul 1;8(7):e09925.
CrossRef - Nemade SM, Kakad SP, Kshirsagar SJ, Padole TR. Development of nanoemulsion of antiviral drug for brain targeting in the treatment of neuro-AIDS. Beni-Suef University Journal of Basic and Applied Sciences. 2022 Dec;11(1):1-0.
CrossRef - Bergonzi MC, Bilia AR, Landucci E. Applications of innovative technologies to the delivery of antipsychotics. Drug Discovery Today. 2022 Feb 1;27(2):401-21.
CrossRef - Gadhave D, Gupta A, Khot S, Tagalpallewar A, Kokare C. Nose-to-brain delivery of paliperidone palmitate poloxamer-guar gum nanogel: Formulation, optimization and pharmacological studies in rats. InAnnales Pharmaceutiques Françaises 2023 Mar 1 (Vol. 81, No. 2, pp. 315-333). Elsevier Masson.
CrossRef
Abbreviations
BBB-Blood Brain Barrier
CNS-Central Nervous System
IN-Intranasal
FTIR- Fourier transform infrared
RPM- Revolutions per minute
q.s- Quantum satis
PDI-Polydispersity index
DSC-Differential scanning calorimetry

This work is licensed under a Creative Commons Attribution 4.0 International License.





