Manuscript accepted on : 30-09-2024
Published online on: 17-10-2024
Plagiarism Check: Yes
Reviewed by: Dr Ana Catarina Matos
Second Review by: Dr. Shailendra Yadav
Final Approval by: Dr. Chateen Izaddin Ali Pambuk
Anutosh Patra1 , Abhishek Samanta2
, Abhishek Samanta2 , Anindita Chakraborty3
, Anindita Chakraborty3 , Nandan Bhattacharyya4*
, Nandan Bhattacharyya4* and Anutosh Patra1
and Anutosh Patra1
1Department of Physiology, PanskuraBanamaliCollege(Autonomous), P.O.-Panskura R.S., West Bengal, India
2Department of Zoology, PanskuraBanamali College, P.O., Panskura R.S., West Bengal, India.
3Department of Stress biology, UGC DAE, Consortium for Scientific Research, Kolkata Centre, Sector III, LB-8, Bidhan Nagar, Kolkata, West Bangal, India.
4Department of Biotechnology, PanskuraBanamali College (Autonomous), P.O.-Panskura R.S., West Bengal, India
Corresponding Author E-mail: bhattacharyya_nandan@rediffmail.com
ABSTRACT: Background: Exposure to mutagens causes DNA damage, which, if not repaired properly, can lead to diseases like cancer. Ovarian cancer is a major concern for women globally, including in India, as it is often diagnosed at an advanced stage, making treatment more challenging. Recent research implicates DNA repair proteins like DNA polymerase β (Pol β) in cancer development, emphasising the need to understand these pathways for targeted therapy. This study uses bibliometric analysis to explore ovarian cancer research and DNA repair pathways, providing insights for future research and treatment. Materials and Methods: Data from 37,539 articles related to cancer, ovarian cancer, DNA polymerase β, DNA repair pathways, and inhibitors were analysed from the Dimensions database. Publication distribution, national cooperation, leading authors, and research trends were examined. Results: Variations in publication distribution were observed across journals, with notable contributions from countries like Germany, Canada, and the Netherlands. Prolific authors and institutions were identified, shedding light on the global academic landscape. Co-occurrence analysis revealed thematic clusters, including pathophysiology, cancer risk associations, therapeutic targets, and genomic research. Conclusion: This bibliometric analysis offers valuable insights into ovarian cancer research and DNA repair pathways. It highlights the importance of targeting DNA repair mechanisms in cancer therapy and suggests opportunities for collaboration and personalised medicine. Identifying key trends and future directions aids in advancing our understanding and treatment of ovarian cancer, aiming to improve patient outcomes.
KEYWORDS: Bibliometric analysis; Cancer therapy; DNA polymerase beta; DNA repair pathways; DNA polymerase beta inhibitors; Ovarian cancer
| Copy the following to cite this article: Patra A, Samanta A, Chakraborty A, Bhattacharyya N. Advancements in Ovarian Cancer Research: Targeting DNA Repair Mechanisms and the Role of DNA Polymerase β Inhibitors. Biotech Res Asia 2024;21(4). |
| Copy the following to cite this URL: Patra A, Samanta A, Chakraborty A, Bhattacharyya N. Advancements in Ovarian Cancer Research: Targeting DNA Repair Mechanisms and the Role of DNA Polymerase β Inhibitors. Biotech Res Asia 2024;21(4). Available from: https://bit.ly/48iqoNu |
Introduction
The human body is continuously exposed to mutagens that cause DNA damage, which is swiftly countered by sophisticated DNA repair mechanisms essential for preserving genomic stability. This dynamic balance between DNA damage and repair has critical implications for aging and diseases like cancer. Numerous cancers, including those of the colon, lung, breast, bladder, and oesophagus, have been linked to mutations in DNA repair proteins such as DNA polymerase β (Pol β)¹. Recent research has increasingly focused on Pol β mutations in ovarian cancer, a major global health concern, particularly in India, where it ranks third in cancer frequency and eighth in overall cancer prevalence²˒³. Ovarian cancer is a significant contributor to cancer-related mortality worldwide, largely due to late-stage diagnosis, which severely limits treatment options. In India, ovarian cancer accounts for 3.44% of all cancer cases and 3.34% of cancer-related deaths⁴˒⁶. While early detection at Stage I yields a promising five-year survival rate of 94%, the majority of cases are diagnosed at Stages III and IV, where the survival rate drops to 28%⁶. Globally, this underscores the need for improved diagnostic methods and more effective treatments. Studies suggest that defects in DNA repair mechanisms can increase tumor sensitivity to chemotherapy, as evidenced by the efficacy of PARP inhibitors in BRCA1-deficient cancers⁸˒⁹. In addition to PARP inhibitors, other DNA repair enzymes, such as glycosylases, phosphodiesterases, and Pol β, are being actively investigated as therapeutic targets. Inhibitors of these enzymes, particularly when combined with DNA-damaging agents like radiation or alkylating agents, have demonstrated potential in selectively inducing cancer cell death by exploiting cancer-specific DNA repair deficiencies⁹˒¹⁰. This therapeutic approach offers a promising strategy to improve the efficacy of conventional treatments for ovarian cancer.
Ovarian cancer remains a formidable health issue worldwide, including in India, where advanced-stage diagnosis presents significant treatment challenges. Enhanced understanding of the molecular mechanisms underlying DNA repair deficiencies in ovarian cancer, combined with the development of targeted therapies, holds potential for improving outcomes for affected women. Epithelial ovarian cancer is a heterogeneous disease with five major subtypes: high-grade serous, low-grade serous, endometrioid, clear cell, and mucinous, with high-grade serous being the most prevalent. The extensive genetic instability and lack of prominent driver mutations complicate molecular profiling in many forms of the disease⁷˒¹¹. While homologous recombination defects are linked to improved responses to platinum-based and PARP inhibitor therapies, leveraging deficiencies in other DNA repair pathways offers a promising avenue for treatment stratification¹¹˒¹². Mammalian cells rely on five primary DNA repair mechanisms: mismatch repair (MMR), base excision repair (BER), nucleotide excision repair (NER) for single-strand breaks, and homologous recombination (HR) and non-homologous end joining (NHEJ) for double-strand breaks¹³˒¹⁴˒¹⁵. In cancer cells, replication stress often leads to genomic instability, compromising genomic integrity⁷. Cancer therapies aim to exacerbate DNA damage or impair DNA damage repair (DDR), thereby inducing catastrophic damage and cell death. DDR inhibitors, particularly those targeting cancer cells, offer better tolerability than conventional chemotherapy, as they minimize damage to normal cells¹⁶.
Overexpression of Pol β in ovarian cancer has been linked to pro-tumorigenic effects, as it may enhance the cancer cells’ ability to repair DNA damage, promoting survival and resistance to chemotherapy. This heightened repair capability can lead to increased genomic instability and tumor aggressiveness. Consequently, inhibiting Pol β could sensitize ovarian cancer cells to DNA-damaging agents, enhancing therapeutic efficacy by disrupting their repair mechanisms. Therefore, targeting Pol β represents a promising strategy for improving treatment outcomes in ovarian cancer patients⁸˒⁹. Targeting DNA repair pathways, especially in cancer cells, enhances the effectiveness of DNA-damaging agents. BER is a critical pathway that repairs damaged DNA bases caused by agents like bleomycin, cisplatin, and alkylating agents. Pol β plays a pivotal role in BER by excising 5′-terminal deoxyribose phosphate (dRP) groups from incised abasic sites and filling in the gaps through template-directed nucleotide incorporation¹⁸. Inhibiting Pol β has emerged as a promising strategy in cancer therapy, as it could enhance the cytotoxicity of DNA-damaging agents. Several Pol β inhibitors, targeting both its polymerase and lyase activities, have shown potential in amplifying the cytotoxic effects of such agents, though more research is needed to clarify their precise mechanisms in cancer cells¹⁹˒²⁰.
Bibliometrics, the statistical and mathematical analysis of publications, is an invaluable tool for identifying research trends and emerging areas of study. A bibliometric analysis of articles from the Dimensions Collection database (2015-2024) was conducted to explore the current landscape of ovarian cancer research, focusing on DNA polymerase β, repair pathways, and inhibitors. This study aims to identify emerging trends, key contributors, and potential future research directions in the field of Pol β mutations and inhibitors relevant to ovarian cancer therapy.
Subjects and Methods
Data Retrieval Strategy
To ensure data accuracy, we utilized the Dimensions database, spanning the index period from January 1, 2015, to March 4, 2024²¹˒²². A refined search query incorporating key terms such as “cancer, ovarian cancer, DNA polymerase beta, DNA polymerase beta inhibitors, DNA repair pathway, polymorphism, SNP, variants, and truncated forms” was employed to identify relevant literature. This search yielded 37,539 articles, providing a robust and comprehensive dataset for analysis. Here are the search results:
https://app.dimensions.ai/discover/publication?search_mode=content&search_text=cancer%2 C%20ovarian%20cancer%2C%20dna%20polymerase%20beta%2C%20dna%20polymerase%20beta%20inhibitors%2C%20dna%20repair%20pathway%2C%20Polymorphism%2C%20SNP%2C%20variants%2C%20Truncated%20Forms&search_type=kws&search_field=full_search.
Data Processing Strategy
A comprehensive publication analysis was conducted to evaluate the distribution of research outputs, national collaborations, and the most productive institutions in ovarian cancer research. This analysis included a total of 37,539 relevant articles, providing valuable insights into the publication landscape of the field (Table 1). Subsequently, co-citation analysis was performed using VOSviewer, wherein the full records and cited reference data from these articles were imported to examine co-citation patterns among cited sources, authors, and references²³. This analysis illuminated the foundational knowledge in the areas of ovarian cancer, DNA pol β mutations, and inhibitors.In addition, a co-occurrence analysis of author keywords was carried out using VOSviewer, facilitating the identification of co-occurring keywords and clustering patterns. This approach revealed key research hotspots and emerging directions within the domains of ovarian cancer, DNA pol β mutations, and DNA repair protein inhibitors²⁴–28.
Table 1: Journal with Ovarian Cancer Research and DNA Repair Pathways Publication and Citation Analysis
| Name | Publications | Citations | Citations mean |
| Medicine and Science in Sports and Exercise | 222 | 46 | 0.21 |
| Journal of Pediatric Gastroenterology and Nutrition | 202 | 67 | 0.33 |
| Arthritis Research and Therapy | 175 | 107 | 0.61 |
| Pancreas | 170 | 49 | 0.29 |
| Methods in Molecular Biology | 141 | 1186 | 841 |
| Advances in Experimental Medicine and Biology | 131 | 1646 | 12.56 |
| Breast Cancer Research | 108 | 83 | 0.77 |
| Epilepsia | 61 | 73 | 1.2 |
| Epidemiology | 50 | 20 | 0.4 |
| Journal of Bone and Mineral Research | 33 | 121 | 3.67 |
 |
Figure 1: Flow chart of thalassemia research evolution with time. |
Results
Publications Analysis
Publication Distribution Analysis
The publication rates vary significantly among the journals analyzed, reflecting differences in research output. Notably, journals such as “Methods in Molecular Biology” and “Advances in Experimental Medicine and Biology” demonstrate higher publication rates, with 141 and 131 articles published, respectively. In contrast, other journals, including the “Journal of Thrombosis and Haemostasis” and “RNA Technologies,” have considerably lower outputs, each contributing only 13 publications. This disparity underscores the varying focus and capacity of journals in disseminating research related to ovarian cancer and associated fields.
In terms of mean citations per publication, there is considerable variation among the journals. Journals like “Physiological Reviews” and “Pharmacological Reviews” have exceptionally high mean citations, with 1063.71 and 277.71 citations respectively, indicating their significant influence and impact in their respective fields. On the other hand, journals like “Arthritis Research and Therapy” and “Cancers” also have relatively high mean citations, with 0.61 and 46.45 citations respectively.
National Cooperation Analysis
The bibliographic analysis provides insights into the geographic distribution of scholarly contributions among the authors listed. Among the countries represented, Germany emerges as a notable hub of academic activity, with multiple authors hailing from this region. Authors such as Tobias Görge, Stefan Werner Schneider, and Christoph Plass, all based in Germany, have contributed significantly to the body of literature, collectively producing 41 publications. Despite their geographic proximity, their citation impact varies, indicating the diversity of research output and its reception within the German academic landscape.
Canada, represented by Alexander K C Leung, showcases a high publication output with 33 publications. However, the mean citations per publication for Leung’s work remain relatively low, suggesting a potential gap between publication volume and citation impact within the Canadian academic context.
Other countries such as the Netherlands, represented by Hugo Ten Cate, and Japan, represented by Tetsuro Kokubo, demonstrate significant scholarly activity, albeit with varying levels of citation impact. Notably, authors from Qatar and Uruguay, such as Martin S Steinhoff and Manuel Ibarra respectively, contribute to the global academic discourse, highlighting the diversity of perspectives and research contributions across different regions.
New Zealand and Switzerland, represented by Christine M Morris and Steffen Gay respectively, also demonstrate active scholarly engagement, albeit with smaller publication numbers compared to other countries. This analysis underscores the global nature of academic research and the diverse contributions made by scholars from various geographic regions.
Leading Author Analysis
The publication records of several authors provide insight into their scholarly contributions and the impact of their work within their respective fields. Among these authors, Alexander K C Leung stands out with an extensive publication history, having contributed to a total of 33 publications. However, despite this prolific output, the mean citations per publication for Leung’s work remain relatively low, indicating a modest level of citation impact (Fig.2).
On the other hand, authors like Tobias Görge, Stefan Werner Schneider, and Martin S Steinhoff have each amassed 15 publications, demonstrating a consistent level of scholarly activity. Interestingly, despite having the same number of publications, their citation records vary, with Görge receiving 5 citations and Schneider and Steinhoff also receiving 5 citations each. This suggests that the impact of their work, as measured by citations, is comparable.
Hugo Ten Cate and Tetsuro Kokubo, although contributing significantly to the body of literature with 19 and 13 publications respectively, have yet to receive citations for their work, indicating that their research may not have garnered significant attention within the academic community.
Meanwhile, authors such as Christoph Plass, Steffen Gay, and Christine M Morris have received citations for their contributions, albeit at a modest level. Plass, with 11 publications, has received 1 citation, while Gay and Morris, with 10 and 9 publications respectively, have also received 1 citation each.
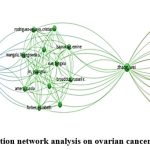 |
Figure 2: Authors corelation network analysis on ovarian cancer and associated studies. |
Analysis of the Most Productive Institutions
The bibliographic analysis provides insights into the academic contributions of researchers affiliated with various organisations across different countries. Among the organisations listed, notable academic institutions and medical centres are represented, each contributing to the global body of scholarly literature.(Fig.3)
Researchers such as Alexander K C Leung, affiliated with Alberta Children’s Hospital in Canada, showcase a substantial publication output, with 33 publications. However, the mean citations per publication for Leung’s work remain relatively low, indicating a potential gap between publication volume and citation impact within the Canadian academic context.
Hugo Ten Cate, associated with Maastricht University Medical Centre in the Netherlands, demonstrates active scholarly engagement with 19 publications. However, the citation impact for Ten Cate’s work appears to be minimal, suggesting varying levels of recognition within the Dutch academic landscape.
In Germany, researchers from leading institutions, including University Hospital Münster and University Medical Center Hamburg-Eppendorf, significantly contribute to the scholarly literature. Notable figures such as Tobias Görge and Stefan Werner Schneider exemplify this trend, achieving both high publication outputs and substantial citation impacts. Their contributions reflect the robust academic environment in Germany and underscore the country’s pivotal role in advancing research on ovarian cancer and related fields.
Martin S Steinhoff, affiliated with Hamad Medical Corporation in Qatar, represents scholarly engagement in the Middle East, contributing to the global discourse with 15 publications. Similarly, researchers from Yokohama City University in Japan, such as Tetsuro Kokubo, demonstrate active participation in academic research despite a lower citation impact.
Christoph Plass, associated with the German Cancer Research Canter, exhibits notable scholarly contributions with 11 publications and a moderate citation impact, highlighting the institution’s role in advancing cancer research.
Other researchers, including Manuel Ibarra from the University of the Republic in Uruguay, Steffen Gay from the University Hospital of Zurich in Switzerland, and Christine M Morris from the University of Otago in New Zealand, also contribute to the global academic landscape with their research endeavours. This analysis underscores the diverse array of institutions and researchers driving scholarly progress across different countries.
 |
Figure 3: Evolution network on organizational contribution analysis. |
Co-occurrence Analysis
Prior to conducting the co-occurrence analysis, the keywords in the dataset underwent term consolidation in VOSviewer, which involved merging synonymous terms and addressing variations in spelling, including singular and plural forms (Fig. 4). From a total of 9,485 keywords, the top 167 were selected based on their total link strength (TLS) for visual analysis. Figure 4 (Fig. 4) illustrates the co-occurrence patterns of these high-frequency author keywords in the context of ovarian cancer, DNA pol β mutations, and inhibitors research. The keywords were organized into five distinct clusters, each represented by a different color, and sorted within each cluster according to their TLS. This analysis identified four primary research hotspots: 1) Insights into Pathophysiology and Therapeutic Modulation, 2) DNA Repair and Cancer Risk Associations, 3) Cancer Pathogenesis and Therapeutic Targets, and 4) Genomic Research Landscape.
 |
Figure 4: Cooccurrence analysis of clusters, where green, yellow, red and blue determined the Insights into Pathophysiology and Therapeutic Modulation, DNA Repair and Cancer Risk Associations, |
Discussion
Research Trends from the Perspective of Time
The bibliometric analysis of ovarian cancer research, focusing on Pol β mutations and DNA Pol β inhibitors29, shows fluctuating publication trends between 2015 and 2024. In 2018, there was a notable peak with 3,845 publications, indicating a significant focus on the topic during that period. This was followed by a decrease in publications in 2019, with only 275 publications. Subsequently, there was a further decline in 2020 and 2021, with 219 and 1,406 publications respectively. However, the trend shifted again in 2022, showing an increase in publications to 1,124. This trend continued with a slight decrease in 2023, with 195 publications. Finally, the study was conducted until March 2024, encompassing only the initial three months of that year, during which there was a substantial drop in publications, totalling just 27.
Cluster Focusing
Cluster analysis, particularly co-occurrence analysis, offers valuable insights into the thematic landscape of ovarian cancer research, Pol β mutations, and inhibitors by identifying patterns in keyword co-occurrence and clustering. This method uncovers underlying themes, research hotspots, and emerging directions in the field.
Cluster 1
“Insights into Pathophysiology and Therapeutic Modulation” focuses on the underlying mechanisms of disease and therapeutic interventions⁵⁻⁸. It explores physiological processes such as inflammation, oxidative stress, cell proliferation, and differentiation, and investigates how specific pathways are modulated through inhibition or activation of enzymes, transcription factors, or genes. This cluster highlights advancements in understanding disease pathophysiology and the development of novel treatment strategies.
Cluster 2
“DNA Repair and Cancer Risk Associations” examines the relationship between DNA repair mechanisms and cancer susceptibility⁹⁻¹². It centers on keywords like “DNA repair,” “mutation,” and “DNA damage,” exploring how alterations in DNA repair pathways contribute to cancer development, particularly in breast, colorectal, and lung cancers. It also discusses the role of genes such as BRCA1 and BRCA2 and the impact of single nucleotide polymorphisms (SNPs) on cancer risk. The cluster underscores the importance of genetic predisposition and DNA repair deficiencies in understanding cancer risk across different types of cancer.
Cluster 3
“Cancer Pathogenesis and Therapeutic Targets” delves into cancer development and treatment challenges, with a focus on carcinogenesis, chemotherapy, drug resistance, and genomic instability¹³⁻¹⁶. It emphasizes the need for novel therapeutic strategies to address tumor growth, metastasis, and drug resistance, with particular attention to terms like “metastasis,” “proliferation,” and “tumorigenesis.” The cluster stresses the critical need for targeted therapies to overcome resistance and improve treatment efficacy in cancer management.
Cluster 4
“Genomic Research Landscape” reflects the diversity and breadth of genomic research in cancer biology¹⁷⁻²⁰. Covering topics such as genome analysis, evolutionary genetics, technological advancements, and research trends, this cluster underscores the expanding scope of genomic investigations and their role in advancing cancer research.
Together, these clusters provide a comprehensive overview of the research landscape surrounding ovarian cancer, Pol β mutations, and inhibitors. By identifying key themes, emerging trends, and research hotspots, these findings offer valuable insights for researchers, clinicians, and policymakers aiming to advance the understanding of cancer pathogenesis and improve therapeutic strategies, ultimately enhancing patient outcomes.
Future Directions and Recommendations
The bibliometric analysis of ovarian cancer research, specifically focusing on DNA polymerase β (Pol β) mutations, DNA repair pathways, and associated DNA Pol β inhibitors, provides a comprehensive assessment of the current scientific landscape30. Through the identification of research trends and emerging directions via cluster and co-occurrence analyses, the study offers a roadmap for addressing critical knowledge gaps and guiding future research. Notably, the analysis underscores the potential of targeting DNA repair pathways for therapeutic interventions, emphasizing the relevance of these pathways in personalized medicine and the development of novel, targeted cancer treatments. The co-occurrence analysis of keywords reveals promising areas for exploration, including novel biomarkers and therapeutic targets within ovarian cancer and DNA repair mechanisms. Additionally, the analysis of global collaborations and leading authors highlights opportunities for fostering international research partnerships that can pool resources and expertise to accelerate advancements in this field.
However, significant gaps remain, particularly regarding the mechanistic understanding of Pol β mutations and their specific role in ovarian cancer progression. There is a marked need for further studies focusing on the functional implications of these mutations and their impact on DNA repair pathways. This includes conducting robust mechanistic studies and clinical trials to evaluate the efficacy of Pol β inhibitors and other pathway-targeting agents in overcoming chemo resistance and improving treatment outcomes. To bridge these gaps, the study recommends a concerted effort in mechanistic investigations of Pol β mutations31 and the initiation of clinical trials evaluating DNA repair pathway inhibitors. Furthermore, enhancing international collaboration among researchers can expedite the discovery of novel therapeutic strategies and biomarkers, ultimately improving the clinical management of ovarian cancer. This approach holds promise for advancing the development of personalized treatment options aimed at enhancing efficacy and patient outcomes.
Conclusion
In conclusion, our bibliometric analysis offers valuable insights into ovarian cancer research and DNA repair pathways. By examining publication distribution, national cooperation, leading authors, and research trends, we’ve identified key hotspots and future directions. Our findings underscore the importance of targeting DNA repair pathways in cancer therapy and highlight opportunities for collaboration and personalised medicine approaches. Ultimately, this study provides a framework for advancing our understanding and treatment of ovarian cancer, aiming to improve patient outcomes through innovative research strategies.
Acknowledgment
The author Anutosh Patra expresses his thanks to UGC DAE, Consortium For Scientific Research, Kolkata Centre (UGC DAE CSR KC) and Panskura Banamali College (Autonomous).The authors also acknowledge Dr. Kolyani Khanra, Dr. Narayanan Chandra Jana and Dr. Indranil Choudhuri for miscellaneous help.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethical approval
The study followed the ethical guidelines of the institutional review board of Panskura Banamali College(Autonomous).
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Authors’ Contributions
All authors contributed to the conception and design of this study.
Anutosh Patra and Abhishek Samanta : designed the study, collected the data, and prepared the manuscript.
Anindita Chakraborty : prepared the manuscript.
Nandan Bhattacharyya : designed and supervised the study and prepared the final manuscript.
All of the authors gave final approval for this version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work is appropriately investigated and resolved.
References
- Yoshizawa, K., Jelezcova, E., Brown, A. R., Foley, J. F., Nyska, A., Cui, X., Hofseth, L. J., Maronpot, R. M., Wilson, S. H., Sepulveda, A. R., & Sobol, R. W. (2009). Gastrointestinal Hyperplasia with Altered Expression of DNA Polymerase β. PLoS ONE, 4(8), e6493. https://doi.org/10.1371/ journal.pone. 0006493
CrossRef - Chen, C., Lin, C.-J., Pei, Y.-C., Ma, D., Liao, L., Li, S.-Y., Fan, L., Di, G.-H., Wu, S.-Y., Liu, X.-Y., Wang, Y.-J., Hong, Q., Zhang, G.-L., Xu, L.-L., Li, B.-B., Huang, W., Shi, J.-X., Jiang, Y.-Z., Hu, X., & Shao, Z.-M. (2023). Comprehensive genomic profiling of breast cancers characterizes germline-somatic mutation interactions mediating therapeutic vulnerabilities. Cell Discovery, 9(1), 125. https://doi.org/10.1038/s41421-023-00614-3
CrossRef - Youssef, A. S. E.-D., Zekri, A. R. N., Mohanad, M., Loutfy, S. A., Abdel Fattah, N. F., Elberry, M. H., El Leithy, A. A., El-Touny, A., Rabie, A. S., Shalaby, M., Hanafy, A., Lotfy, M. M., El-sisi, E. R., El-Sayyad, G. S., & Nassar, A. (2023). Deleterious and ethnic-related BRCA1/2 mutations in tissue and blood of Egyptian colorectal cancer patients and its correlation with human papillomavirus. Clinical and Experimental Medicine, 23(8), 5063–5088. https://doi.org/10.1007/s10238-023-01207-w
CrossRef - Chaturvedi, M., Krishnan, S., Das, P., Sudarshan, K. L., Stephen, S., Monesh, V., & Mathur, P. (2023). Descriptive Epidemiology of Ovarian Cancers in India: A Report from National Cancer Registry Programme. Indian Journal of Gynecologic Oncology, 21(1), 25. https://doi.org/10.1007/s40944-022-00694-1
CrossRef - Bisht, D., Arora, A., & Sachan, M. (2022). Role of DNA De-methylation intermediate ‘5-hydroxymethylcytosine’ in ovarian cancer management: A comprehensive review. Biomedicine & Pharmacotherapy, 155, 113674. https://doi.org/10.1016/j.biopha.2022.113674
CrossRef - K. Gaikwad, M., Upadhye, M., Borchate, D., &Jankar, N. (2023). Impact of Hazardous Chemical compounds on Reproductive System Reported in Sanitary Products. Research Journal of Pharmacology and Pharmacodynamics, 112–118. https://doi.org/10.52711/2321-5836.2023.00021
CrossRef - Crinò, L., Weder, W., Van Meerbeeck, J., & Felip, E. (2010). Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 21, v103–v115. https://doi.org/10.1093/annonc/mdq207
CrossRef - Baradács, I., Teutsch, B., Váradi, A., Bilá, A., Vincze, Á., Hegyi, P., Fazekas, T., Komoróczy, B., Nyirády, P., Ács, N., Bánhidy, F., & Lintner, B. (2024). PARP inhibitor era in ovarian cancer treatment: A systematic review and meta-analysis of randomized controlled trials. Journal of Ovarian Research, 17(1), 53. https://doi.org/10.1186/s13048-024-01362-y
CrossRef - Grundy, G. J., & Parsons, J. L. (2020). Base excision repair and its implications to cancer therapy. Essays in Biochemistry, 64(5), 831–843. https://doi.org/10.1042/EBC20200013
CrossRef - Mirza, M. R., Coleman, R. L., González-Martín, A., Moore, K. N., Colombo, N., Ray-Coquard, I., &Pignata, S. (2020). The forefront of ovarian cancer therapy: Update on PARP inhibitors. Annals of Oncology, 31(9), 1148–1159. https://doi.org/10.1016/j.annonc.2020.06.004
CrossRef
- Ronchi, S., Facchi, S., Di Lauro, E., Libera, L., Carnevali, I. W., Zefiro, F., Alexandrova, E., Rizzo, F., Sessa, F., &Tibiletti, M. G. (2024). Immunohistochemical and molecular pattern of p53 in epithelial ovarian cancers negative for germline BRCA1/2 variants. Pathology – Research and Practice, 255, 155183. https://doi.org/10.1016/j.prp.2024.155183
CrossRef - Hong, W., Zhang, Y., Wang, S., Zheng, D., Hsu, S., Zhou, J., Fan, J., Zeng, Z., Wang, N., Ding, Z., Yu, M., Gao, Q., & Du, S. (2024). Deciphering the immune modulation through deep transcriptomic profiling and therapeutic implications of DNA damage repair pattern in hepatocellular carcinoma. Cancer Letters, 582, 216594. https://doi.org/10.1016/j.canlet.2023.216594
CrossRef - Chatterjee, N., & Walker, G. C. (2017). Mechanisms of DNA damage, repair, and mutagenesis. Environmental and Molecular Mutagenesis, 58(5), 235–263. https://doi.org/10.1002/em.22087
CrossRef - Groelly, F. J., Fawkes, M., Dagg, R. A., Blackford, A. N., &Tarsounas, M. (2023). Targeting DNA damage response pathways in cancer. Nature Reviews Cancer, 23(2), 78–94. https://doi.org/10.1038/s41568-022-00535-5
CrossRef - Kamat, A., & Badrinarayanan, A. (2023). SOS-independent bacterial DNA damage responses: Diverse mechanisms, unifying function. Current Opinion in Microbiology, 73, 102323. https://doi.org/10.1016/j.mib.2023.102323
CrossRef - Sánchez-Chapul, L., Santamaría, A., Aschner, M., Ke, T., Tinkov, A. A., Túnez, I., Osorio-Rico, L., Galván-Arzate, S., & Rangel-López, E. (2023). Thallium-induced DNA damage, genetic, and epigenetic alterations. Frontiers in Genetics, 14, 1168713. https://doi.org/10.3389/fgene.2023.1 168713
CrossRef - Chang, T., Lian, Z., Ma, S., Liang, Z., Ma, X., Wen, X., Wang, Y., & Liu, R. (2023). Combination with vorinostat enhances the antitumor activity of cisplatin in castration‐resistant prostate cancer by inhibiting DNA damage repair pathway and detoxification of GSH. The Prostate, 83(5), 470–486. https://doi.org/10.1002/pros.24479
CrossRef - Li, X., Li, Y., Zhao, Z., Miao, N., Liu, G., Deng, L., Wei, S., & Hou, J. (2023). Immunogenicity of small‐cell lung cancer associates with STING pathway activation and is enhanced by ATR and TOP1 inhibition. Cancer Medicine, 12(4), 4864–4881. https://doi.org/10.1002/cam4.5109
CrossRef - Melia, E., & Parsons, J. L. (2023). DNA damage and repair dependencies of ionising radiation modalities. Bioscience Reports, 43(10), BSR20222586. https://doi.org/10.1042/BSR20222586
CrossRef - Rajendra, E., Grande, D., Mason, B., Di Marcantonio, D., Armstrong, L., Hewitt, G., Elinati, E., Galbiati, A., Boulton, S. J., Heald, R. A., Smith, G. C. M., & Robinson, H. M. R. (2024). Quantitative, titratable and high-throughput reporter assays to measure DNA double strand break repair activity in cells. Nucleic Acids Research, 52(4), 1736–1752. https://doi.org/10.1093/nar/gkad1196
CrossRef - Kulkanjanapiban, P., &Silwattananusarn, T. (2022). Comparative analysis of Dimensions and Scopus bibliographic data sources: An approach to university research productivity. International Journal of Electrical and Computer Engineering (IJECE), 12(1), 706. https://doi.org/10.11591/ijece.v12i1.pp706-720
CrossRef - Delgado-Quirós, L., & Ortega, J. L. (2024). Completeness degree of publication metadata in eight free-access scholarly databases. Quantitative Science Studies, 5(1), 31–49. https://doi.org/10.1162/ qss_a_00286
CrossRef - Guerra, J. M., Deza, C. S., Sánchez, D. T., & León, T. R. (2024). Collaborative Technologies to Optimize Scientific Production in High-Quality Bibliographical Databases Worth. Social Science and Humanities Journal, 8(02), 34468–34477. https://doi.org/10.18535/sshj.v8i01.919
CrossRef - Cocomazzi, G., Del Pup, L., Contu, V., Maggio, G., Parmegiani, L., Ciampaglia, W., De Ruvo, D., Faioli, R., Maglione, A., Baldini, G. M., Baldini, D., & Pazienza, V. (2024). Gynecological Cancers and Microbiota Dynamics: Insights into Pathogenesis and Therapy. International Journal of Molecular Sciences, 25(4), 2237. https://doi.org/10.3390/ijms25042237
CrossRef - Chen, B., Zhao, L., Yang, R., & Xu, T. (2024a). New insights about endometriosis-associated ovarian cancer: Pathogenesis, risk factors, prediction and diagnosis and treatment. Frontiers in Oncology, 14, 1329133. https://doi.org/10.3389/fonc.2024.1329133
CrossRef - Bhardwaj, V., Zhang, X., Pandey, V., & Garg, M. (2023). Neo-vascularization-based therapeutic perspectives in advanced ovarian cancer. Biochimica et Biophysica Acta (BBA) – Reviews on Cancer, 1878(3), 188888. https://doi.org/10.1016/j.bbcan.2023.188888
CrossRef - Chen, B., Zhao, L., Yang, R., & Xu, T. (2024b). New insights about endometriosis-associated ovarian cancer: Pathogenesis, risk factors, prediction and diagnosis and treatment. Frontiers in Oncology, 14, 1329133. https://doi.org/10.3389/fonc.2024.1329133
CrossRef - Cong, P., Shang, B., Zhang, L., Wu, Z., Wang, Y., Li, J., & Zhang, L. (2024). New insights into the treatment of polycystic ovary syndrome: HKDC1 promotes the growth of ovarian granulocyte cells by regulating mitochondrial function and glycolysis. Journal of Molecular Histology, 55(2), 187–199. https://doi.org/10.1007/s10735-024-10183-8
CrossRef - Fantone, S., Piani, F., Olivieri, F., Rippo, M. R., Sirico, A., Di Simone, N., Marzioni, D., & Tossetta, G. (2024). Role of SLC7A11/xCT in Ovarian Cancer. International Journal of Molecular Sciences, 25(1), 587. https://doi.org/10.3390/ijms25010587
CrossRef - Patra, A., Choudhuri, I., Paria, P., Samanta, A., Khanra, K., & Chakraborty, A. (2024). Phytochemicals as Potential DNA Polymerase β Inhibitors for Targeted Ovarian Cancer Therapy: An In-silico Approach. Biosciences Biotechnology Research Asia, 21(2), 617-631.
CrossRef - Patra, A., Pan, P., & Bhattacharyya, N. (2024). Error-prone DNA synthesis and accumulation of single nucleotide gaps by DNA Polymerase β leads to cancer: A bibliometric analysis. African Journal of Biological Sciences, 6(13), 2995-3011. https://doi.org/10.48047/AFJBS.6.13.2024.2995-3011
- Patra, A., Nag, A., Chakraborty, A., & Bhattacharyya, N. (2022). PA1 cells containing a truncated DNA polymerase β protein are more sensitive to gamma radiation. Radiation Oncology Journal, 40(1), 66.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





