Manuscript accepted on : 03-02-2025
Published online on: 11-02-2025
Plagiarism Check: Yes
Reviewed by: Dr. Y. Indira Muzib
Second Review by: Dr. Ahmed Salah
Final Approval by: Dr. Wagih Ghannam
Shivraj Popat Jadhav1* , Sushant Mothabhau Ahire2
, Sushant Mothabhau Ahire2 , Vijay Vithoba Shewale3
, Vijay Vithoba Shewale3 , Chandrashekhar Dinkar Patil4
, Chandrashekhar Dinkar Patil4 , Rahul Yuvraj Pagar5
, Rahul Yuvraj Pagar5 , Deepak Devidas Sonawane1
, Deepak Devidas Sonawane1 and Sunil Kashinath Mahajan2
and Sunil Kashinath Mahajan2
1Department of Pharmaceutics, Divine College of Pharmacy, Satana, Nashik, Maharashtra, India.
2Department of Pharmaceutical Chemistry, Divine College of Pharmacy, Satana, Nashik, Maharashtra, India.
3Department of Pharmacognosy, Divine College of Pharmacy, Satana, Nashik, Maharashtra, India.
4Department of Pharmacology, Divine College of Pharmacy, Satana, Nashik, Maharashtra, India.
5Department of Pharmaceutics, K.B.H.S.S. Trust's Institute of Pharmacy, Malegaon, Nashik, Maharashtra, India.
Corresponding Author E-mail id: shiva.007ind@gmail.com
ABSTRACT: Nifedipine is commonly prescribed for preterm labor, hypertension, and angina pectoris. According to the Biopharmaceutics Classification System (BCS), nifedipine is categorized as a Class II drug due to its low solubility and high permeability. This study aimed to enhance nifedipine's solubility using a co-crystal approach. Co-crystals are multi-component systems composed of a stoichiometric ratio of an active pharmaceutical ingredient (API) and an approved co-former. Through solvent-assisted grinding, nifedipine co-crystals were successfully prepared with glutaric acid, succinic acid, cinnamic acid, and fumaric acid in a 1:1 stoichiometric ratio. The physicochemical properties of the co-crystals, including infrared spectroscopy, melting point, X-ray diffraction, differential scanning calorimetry, and solubility profiles, were compared to those of pure nifedipine. Among the four co-crystals, the nifedipine-fumaric acid (NFD-FA) co-crystal exhibited a remarkable 90-fold improvement in solubility over pure nifedipine. Consequently, the NFD-FA co-crystal was further characterized. Excipient compatibility studies were conducted, and tablets were formulated using the wet granulation method. In-vitro drug release studies revealed that NFD-FA co-crystal tablets achieved a 45% cumulative drug release within 60 minutes, significantly outperforming the marketed formulation, which released only 27% of the drug in the same period. The observed improvement in solubility is likely due to the formation of hydrogen bonds between nifedipine and the fumaric acid co-former, demonstrating the effectiveness of co-crystallization as a strategy for enhancing drug solubility and dissolution.
KEYWORDS: Co-Crystal; Co-Former; Hypertension; Nifedipine; Solubility
| Copy the following to cite this article: Jadhav S. P, Ahire S. M, Shewale V. V, Patil C. D, Pagar R. Y, Sonawane D. D, Mahajan S. K. Formulation of Tablet of Nifedipine Co-Crystal for Enhancement of Solubility and Other Physical Properties. Biotech Res Asia 2025;22(1). |
| Copy the following to cite this URL: Jadhav S. P, Ahire S. M, Shewale V. V, Patil C. D, Pagar R. Y, Sonawane D. D, Mahajan S. K. Formulation of Tablet of Nifedipine Co-Crystal for Enhancement of Solubility and Other Physical Properties. Biotech Res Asia 2025;22(1). Available from: https://bit.ly/3EKEXOQ |
Introduction
Over half of newly created molecules are insoluble in water.1 The development of a novel pharmaceutical requires millions of dollars and a period of ten to fifteen years. Therefore, different techniques are used to increase these compounds’ solubility. Numerous methods2 are employed to increase solubility.
Among the various techniques to address solubility challenges, co-crystals present an innovative and promising solution. Pharmaceutical co-crystals involve the fusion of two distinct molecules in a stoichiometric ratio, resulting in a novel crystal lattice with enhanced physicochemical properties, often surpassing those of the original components.3
Nifedipine (NFD) is a yellow-coloured drug classified under the Biopharmaceutical Classification System (BCS) class II due to its low solubility and high permeability. It is widely used to manage conditions such as angina pectoris, hypertension, and preterm labor.4 Nifedipine (NFD) is an ideal candidate for co-crystal formation due to the presence of proton donor groups such as -NH, which facilitate hydrogen bonding. The selected co-formers—cinnamic acid, glutaric acid, succinic acid, and fumaric acid contain functional groups capable of forming strong hydrogen bonds with NFD, promoting efficient co-crystallization. The co-crystals are prepared using the solvent-assisted grinding method5, which enhances the interaction between NFD and the co-formers to form a stable crystalline structure.
 |
Figure 1: Structure of NFD |
 |
Figure 2: Different Co-formers
|
Material and Methods
Material
A gift sample of nifedipine was kindly provided by Alkem Labs Ltd., Taloja, India. The co-formers and other chemicals were procured from Sudarshan Scientific Laboratories, Nandgaon, Nashik, India. All materials were used as received without further purification.
Method
Preformulation studies of pure nifedipine
Melting point: Theil’s tube method is used to determine the melting point of a substance. In this method, one end of a capillary tube is sealed using a flame. A small amount of the drug is placed at the base of the capillary, and the tube is then inserted into Theil’s tube, where it is secured with a thermometer. The melting point of the active pharmaceutical ingredient (API) is recorded when the substance transitions from solid to liquid.6
Determination of λmax and Preparation of Calibration Curve
Methanol was used to dissolve NFD. The resulting solution was appropriately diluted and scanned using a UV-visible spectrophotometer (UV-1800, Shimadzu, Japan) over the wavelength range of 200–400 nm. For the preparation of the calibration curve, NFD solutions at concentrations of 2, 4, 6, 8, and 10 ppm were prepared, and their absorbances were recorded.
Solubility
A significant amount of NFD was added to 50 mL of water in stoppered glass bottles. The bottles were then shaken for 48 hours on a mechanical shaker at ambient temperature. Afterwards, the saturated solutions were filtered using Whatman filter paper, and the drug concentration was measured with a double-beam UV/Vis spectrophotometer.7
DSC
NFD was analysed using a Mettler DSC. A 5 mg sample of NFD was carefully weighed and sealed in an aluminum pan. The sample was then heated from room temperature to 300°C at a rate of 10°C/min. To maintain an inert atmosphere, pure nitrogen gas was injected at a flow rate of 100 mL/min.8
FTIR
A Pellet of KBr and NFD was compressed in a 1:1 ratio. The FTIR spectrum was obtained using a Bruker FTIR spectrophotometer in 4000 to 400 cm-1 wavelengths.8
XRD
A PANalytical X’Pert Pro with a copper target X-ray tube was used to do the X-ray diffraction (XRD) of the powder sample. For the XRD study, a wavelength of 1.54184 Å was used.9
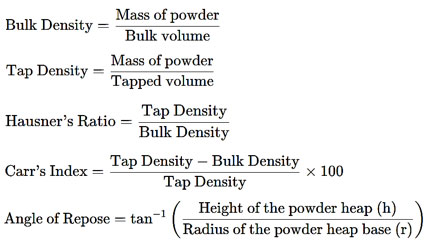
Micromeritic properties
Various micromeritic properties of powder NFD were determined as follows10, 11;
Preparation of Co-crystal
The solvent-assisted grinding method was used to create NFD co-crystals.5 NFD and the co-formers were mixed in equal amounts in a mortar, with two to three droplets of ethyl alcohol added, as shown in Table 1.
Table 1: Preparation of Co-crystal
| Co-crystal | NFD in mg | Co-former in mg |
| NFD- Fumaric acid | 500.00 | 500.00 |
| NFD- Succinic acid | 500.00 | 500.00 |
| NFD- Glutaric acid | 500.00 | 500.00 |
| NFD- Cinnamic acid | 500.00 | 500.00 |
The co-crystals were kept for additional studies in self-sealing plastic bags inside a desiccator.
Evaluation of cocrystal
The co-crystals formed were assessed for several characteristics, including melting point, FTIR, DSC, solubility and XRD following the earlier outlined methodologies.12
Co-crystal Excipient compatibility study
To create stable tablet dosage forms, co-crystals were selected based on previous studies and tested with various excipients in glass vials. Physical observations, including liquefaction, discoloration, and gas formation, were recorded. As shown in Table 2, the samples were removed from the vials and inspected for any odors.13
Table 2: Study of the compatibility of different excipients with co-crystal.
| Co-crystal | Excipient |
| NFD-FA (Fumaric acid) | Magnesium Stearate |
| Avicel PH 102 | |
| PVP K30 | |
| Starch | |
| Lactose |
Formulation of the tablet
Wet granulation was used to create tablets of particular co-crystals (NFD-FA) and excipients.14,15 The weighed excipients were passed through a 40-mesh sieve. The formed co-crystals were then blended with additional excipients. The appropriate amount of starch paste was added to form a damp mass. This damp mass was subsequently dried in an oven set to 40°C and then passed through a 20-mesh sieve. A glidant was added, and the mixture was compressed into 300 mg tablets using a VBtech 8-station tablet punching machine.
Table 3: Formulation of tablet of co-crystal.
| Ingredient | Amount (mg) | Use |
| NFD-FA | 40 | Antihypertensive |
| Lactose | 220 | As a Filler |
| Starch | 20 | As a Binder |
| Avicel (PH 102) | 18 | As a Compression Aid |
| Magnesium stearate | 2 | As a Lubricant |
| 300 mg |
Evaluation of tablet
The tablets were subjected to tests for hardness, weight variation, disintegration time, friability, and in vitro drug release.16
Comparative dissolution studies with marketed tablets
Type II USP dissolution test apparatus was used to analyse the release of the drug of the NFD-FA cocrystal and commercialised nifedipine tablets (Aminifd, Amigoz Life Sciences). The dissolution media consisted of 900 ml of 0.1 N HCl at 37±0.5℃. The paddle speed was set to 50. 5 ml samples were taken every ten minutes and replaced with fresh medium. After 60 minutes of research, samples were filtered and examined with a Shimadzu 1800 UV spectrophotometer.17
Result
Preformulation studies of pure nifedipine
Table 4 indicates the results of the nifedipine preformulation.
Table 4: Pure nifedipine preformulation.
| Test | Observation |
| Melting point | 165 to 167 °C |
| ʎmax (nm) | 237 |
| Tap Density (gm/ml) | 00.85 |
| Bulk density (gm/ml) | 00.69 |
| Saturation Solubility (mg/ml) | 0.0092 |
| % of Carr’s index | 18.82 |
| Angle of repose | 40° |
| Hausner’s ratio | 1.2318 |
Calibration curve
Figure 3 displays the calibration curve for nifedipine, plotted between 2 and 10 ppm concentration.
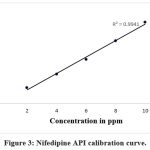 |
Figure 3: Nifedipine API calibration curve. |
DSC
Figure 4 depicts the DSC spectra of pure nifedipine. A discrete peak at 160.80 °C, indicative of the melting point, was seen in the DSC of pure NFD.
FTIR
Figure 5 shows the FTIR spectrum of pure NFD. It has peaks at 3324 cm-1, 1677 cm-1, 3101 cm-1, 1222 cm-1 and 2952 cm-1 corresponding to various functional groups present in NFD.
XRD
Figure 7 shows the XRD structure of Nifedipine. Numerous peaks were seen in the X-ray diffraction of pure nifedipine. This shows that pure nifedipine is crystalline in nature.
Evaluation of Co-crystal
Co-crystals were evaluated for various characteristics, with the results presented in Table 5. All co-crystals melted at temperatures between the melting points of pure nifedipine and the co-crystal former. Previous studies have shown that approximately 51% of co-crystals have a melting point intermediate to those of the purified drug and the co-former. The formulated co-crystals exhibited higher saturation solubility compared to pure nifedipine.
Table 5: Co-crystal preformulation parameter evaluation.
| Parameter | NFD-FA | NFD-SA | NFD-GA | NFD-CA |
| Onset of Melting point | 182 °C | 174 °C | 140 °C | 155 °C |
| Bulk density (gm/ml) | 00.68 | 00.62 | 00.56 | 00.53 |
| Tap Density (gm/ml) | 00.80 | 00.79 | 00.82 | 00.78 |
| Angle of repose | 24° | 31° | 34° | 22° |
| Saturation Solubility (mg/ml) | 0.850 | 0.076 | 0.158 | 0.0590 |
| Hausner’s ratio | 01.17 | 01.27 | 01.46 | 01.34 |
DSC of Nifedipine and NFD-FA
The DSC spectra of NFD-FA exhibited a peak of approximately around 180 ℃.
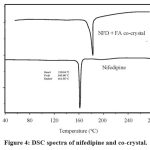 |
Figure 4: DSC spectra of nifedipine and co-crystal. |
FTIR of NFD-FA
FTIR is a valuable technique for studying co-crystal formation. The FTIR spectra of pure nifedipine show a strong N-H stretching peak, whereas the NFD-FA co-crystal spectra display a less intense peak. Figure 5 illustrates the interaction between the -OH group of fumaric acid and the -NH group of nifedipine.
 |
Figure 5: FTIR spectra of Pure nifedipine and nifedipine co-crystal. |
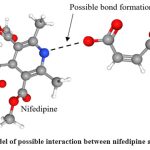 |
Figure 6: 3D model of possible interaction between nifedipine and fumaric acid. |
XRD of NFD-FA
The XRD spectra of the NFD-FA co-crystal exhibit several peaks, hence confirming the crystalline nature of the formed product.
 |
Figure 7: XRD spectra of nifedipine and co-crystal. |
Co-crystal and excipient compatibility study
Following the compatibility investigation, no unusual changes were detected as shown in Table 6.
Table 6: Results of compatibility between co-Crytal and excipients.
| NFD-FACo-crystal
|
Excipient | Liquefaction | Colour Change |
| Lactose | No changes were noted. | ||
| Starch | No changes were noted. | ||
| Magnesium Stearate | No changes were noted. | ||
| Avicel PH 102 | No changes were noted. | ||
| PVP K30 | No changes were noted. | ||
Evaluation of tablets
The results of various parameters of formulated co-crystal tablets are indicated in Table 7.
Table 7: Results of tablet evaluation.
| Test | Observation | Result |
| Hardness | 3 to 5 kg | Test passes |
| Weight variation | The weight of 20 tablets falls between 190 mg to 212 mg | Test passes |
| Time of Disintegration | 10 to 14 min. | Test passes |
| Friability | 0.9 % | Test passes |
| n=3 | ||
Dissolution studies
For drug release studies, NFD-FA co-crystal tablets were compared with marketed tablets. Figure 8 illustrates the drug release patterns of pure nifedipine and NFD-FA co-crystals. After 60 minutes, the marketed formulation showed a cumulative drug release of 27%, whereas the co-crystal tablets demonstrated a drug release of 45%.
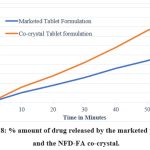 |
Figure 8: % amount of drug released by the marketed product and the NFD-FA co-crystal. |
Discussion
The preformulation studies revealed that nifedipine exhibits passable to fair flow properties and very slightly soluble behavior, as shown in the solubility study. These properties necessitate improvement for enhanced formulation performance. The calibration curve of nifedipine demonstrated a good linearity.
The DSC analysis of pure nifedipine confirmed its crystalline nature with a melting point of 160.80 °C. The FTIR and XRD analyses further supported the crystalline and structural properties of nifedipine. The FTIR spectrum showed characteristic peaks indicative of its chemical structure, while the XRD pattern revealed high crystallinity. A 3324 cm⁻¹ peak in an FTIR (Fourier Transform Infrared) spectrum typically corresponds to the O-H (hydroxyl) or N-H (amine or amide) stretching vibration. This peak is prominent in NFD FTIR, while in Co-Crystal FTIR peak height is reduced. The decrease in the peak height at 3324 cm⁻¹ in the co-crystal FTIR spectrum implies that the hydrogen bonds present in the pure drug are partially replaced or modified by interactions with the co-former in the co-crystal. This is evidence of successful co-crystallization and the formation of a new crystal structure where the functional groups are involved in different molecular interactions compared to the pure drug. Also, The presence of the 2362 cm⁻¹ peak in the nifedipine-fumaric acid co-crystal indicates the formation of a distinct crystal structure with new interactions not present in pure nifedipine. This peak could be associated with, new hydrogen bonding or packing arrangements in the co-crystal, vibrational contributions from fumaric acid, such as a unique stretching mode introduced by the carboxylic groups or lattice vibrations.
The evaluation of co-crystals provided compelling evidence of their enhanced physicochemical properties compared to pure nifedipine. The melting points of the co-crystals were intermediate to those of the drug and co-formers, aligning with previous studies that suggest co-crystals typically exhibit melting points between those of their individual components.18 Among the formulations, NFD-FA exhibited superior solubility (0.850 mg/ml), which was 90 times greater than pure nifedipine. The superior solubility of NFD-FA could be attributed to the formation of stronger hydrogen bonds between the carboxyl groups of fumaric acid and the amino and carbonyl groups of nifedipine. Additionally, its flow properties were improved, as evident from the reduced angle of repose (24°) and Carr’s index (15%), indicating excellent flow characteristics as compared to pure NFD. Improved flow properties ensure uniform die filling, reducing variability in tablet weight, density, and composition during manufacturing. This leads to consistent porosity and prevents over-compression, which enhances tablet disintegration and promotes faster and more reliable dissolution.
The DSC spectra of NFD-FA co-crystals indicated a single peak at approximately at 180 °C, proving the creation of a new crystalline solid. The FTIR analysis of NFD-FA indicated the reduced intensity of the N-H stretching peak, suggesting an interaction between the -OH group of fumaric acid and the -NH group of nifedipine. XRD patterns revealed the creation of a novel crystalline structure, with new peaks confirming the successful formation of co-crystals.
The compatibility study showed no significant changes in physical properties or chemical interactions between the co-crystals and excipients, ensuring stability during formulation development. Evaluation of tablets formulated with NFD-FA co-crystals demonstrated compliance with all pharmacopeial standards, including hardness, weight variation, disintegration, and friability, ensuring their suitability for further use.
The comparative dissolution study with a marketed formulation highlighted the superior drug release profile of the co-crystal tablets. The NFD-FA co-crystal tablets achieved 45% cumulative drug release after 60 minutes compared to 27% for the marketed formulation. The enhanced dissolution rate of NFD-FA co-crystal tablets can be attributed to the combined effects of smaller particle size, improved wettability due to fumaric acid, and the altered crystal lattice structure that facilitates easier drug release. Additionally, the hydrophilic properties of fumaric acid contribute to better solvent penetration and dispersion of nifedipine in the dissolution medium, resulting in faster and more efficient drug release. This improvement in dissolution behavior can enhance the bioavailability of poorly soluble drugs like nifedipine, making co-crystal formation a valuable strategy in pharmaceutical development.
Conclusion
The solvent-assisted grinding method has shown immense potential as a transformative approach for enhancing the physical and chemical properties of BCS class II drugs, which often face challenges related to poor solubility and bioavailability. This study successfully demonstrated the formation of a nifedipine-fumaric acid (NFD-FA) co-crystal, which exhibited significantly improved solubility and faster dissolution rates compared to pure nifedipine. These enhancements are critical for overcoming limitations associated with poorly soluble drugs, offering a pathway to increased therapeutic efficacy and patient compliance. Moreover, the findings underscore the versatility and efficiency of co-crystal technology in drug development, particularly for addressing solubility issues in existing APIs without altering their molecular structure. Future research could explore optimizing co-crystal formation techniques for industrial scalability, evaluating long-term stability, and investigating their performance across diverse pharmaceutical formulations. Additionally, expanding this approach to other APIs with solubility challenges could pave the way for broader applications of co-crystal engineering, revolutionizing drug formulation and delivery in the pharmaceutical industry.
Acknowledgement
The authors are thankful to the IQAC department, Savitribai Phule Pune University for providing the grant to conduct the study under the ASPIRE scheme. The authors are also thankful to Mr. Parag Pathade, and Mr. Tushar Luhade for providing their support during the study.
Funding Source
This project was funded by IQAC, Savitribai Phule Pune University under the ASPIRE scheme (18TEC001108).
Conflict of interest
The authors do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials.
Author Contributions
Shivraj Popat Jadhav: Conceptualization and Basic Research
Shivraj Jadhav, Sushant Ahire and Vijay Shewale: Writing original draft
Chandrashekhar Patil and Rahul Pagar: Proofreading
Sunil Mahajan and Deepak Sonawane: Mentoring.
References
- Paul R., Paul S. Exploration on the drug solubility enhancement in aqueous medium with the help of endo-functionalized molecular tubes: A computational approach. Physical Chemistry Chemical Physics. 2021;23(34):18999-19010.
CrossRef - Savjani K. T., Gajjar A. K., Savjani J. K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharmaceutics. 2012; 2012:1-10.
CrossRef - Vishweshwar P., McMahon J. A., Bis J. A., Zaworotko M. J. Pharmaceutical Co-Crystals. Journal of Pharmaceutical Sciences. 2006;95(3):499-516.
CrossRef - Nader A. M., Quinney S. K., Fadda H. M., Foster D. R. Effect of Gastric Fluid Volume on the In Vitro Dissolution and In Vivo Absorption of BCS Class II Drugs: A Case Study with Nifedipine. American Association of Pharmaceutical Scientists Journal. 2016;18(4):981-988.
CrossRef - Hasmukh M. C., Pooja S. P., Fathy K. B., Koteshwara K., Yogendra N. U. Computational and Experimental Insights in Design and Development of Aceclofenac Co-Crystals. Research Journal of Pharmacy and Technology. 2022; 15(8):3709-3716.
CrossRef - Jadhav S. P., Dhakad P. K., Gupta T., Gilhotra R. Formulation Development and Evaluation of Paliperidone Nanosuspension for Solubility Enhancement. International Journal of Applied Pharmaceutics. 2024:173-181.
CrossRef - Veseli A., Žakelj S., Kristl A. A review of methods for solubility determination in biopharmaceutical drug characterization. Drug Development and Industrial Pharmacy. 2019;45(11):1717-1724.
CrossRef - Tkachenko Y., Niedzielski P. FTIR as a Method for Qualitative Assessment of Solid Samples in Geochemical Research: A Review. Molecules. 2022;27(24):8846.
CrossRef - Garbacz P., Paukszta D., Sikorski A., Wesolowski M. Structural Characterization of Co-Crystals of Chlordiazepoxide with p-Aminobenzoic Acid and Lorazepam with Nicotinamide by DSC, X-ray Diffraction, FTIR and Raman Spectroscopy. Pharmaceutics. 2020;12(7):648.
CrossRef - Jun S. W., Kim M. S., Kim J. S. Preparation and characterization of simvastatin/hydroxypropyl-β-cyclodextrin inclusion complex using supercritical antisolvent (SAS) process. European Journal of Pharmaceutics and Biopharmaceutics. 2007;66(3):413-421.
CrossRef - Chettri A., Subba A., Singh G. P., Bag P. P. Pharmaceutical co-crystals: A green way to enhance drug stability and solubility for improved therapeutic efficacy. Journal of Pharmacy and Pharmacology. 2024;76(1):1-12.
CrossRef - Panzade P., Shendarkar G., Shaikh S., Balmukund R. P. Pharmaceutical Cocrystal of Piroxicam: Design, Formulation and Evaluation. Advanced Pharmaceutical Bulletin. 2017;7(3):399-408.
CrossRef - Kacso I., Rus L. M., Martin F., Miclaus M., Filip X., Dan M. Solid-state compatibility studies of Ketoconazole-Fumaric acid co-crystal with tablet excipients. Journal of Thermal Analysis and Calorimetry. 2021;143(5):3499-3506.
CrossRef - Yamashita H., Sun C. C. Expedited Tablet Formulation Development of a Highly Soluble Carbamazepine Cocrystal Enabled by Precipitation Inhibition in Diffusion Layer. Pharmaceutical Research. 2019;36(6):90.
CrossRef - Ullah M., Hussain I., Sun C. The development of carbamazepine-succinic acid cocrystal tablet formulations with improved in vitro and in vivo Drug Development and Industrial Pharmacy. 2016;42(6):969-976.
CrossRef - Sharma D., Singh M., Kumar D., Singh G., Rathore S. Formulation Development and Evaluation of Fast Disintegrating Tablets of Ambroxol Hydrochloride for Pediatrics- A Novel Approach for Drug Delivery. Indian Journal of Pharmaceutical Education and Research. 2014;48(supplementary):40-48.
CrossRef - Samsodien H., Bapoo M., Doms T. l., Harneker Z., Louw A. S. FTIR, Dissolution and Anti-viral Activity of Nevirapine Co-crystals. Pharmaceutica analytica acta 2017;08(09).
CrossRef - Schultheiss N., Newman A. Pharmaceutical Cocrystals and Their Physicochemical Properties. Crystal Growth & Design. 2009;9(6):2950-2967.
CrossRef
Abbreviations
BCS: Biopharmaceutical Classification System
NFD: Nifedipine
UV/Vis: Ultraviolet-Visible
DSC: Differential Scanning Calorimetry
FTIR: Fourier Transfer Infrared
XRD: X-ray Diffraction

This work is licensed under a Creative Commons Attribution 4.0 International License.





