How to Cite | Publication History | PlumX Article Matrix
C. Uma* and V. K. Gopalakrishnan
Department of Biochemistry, Karpagam Arts and Science College, Coimbatore - 641 021 India.
ABSTRACT: Invertase production by Cladosporium sp., at varying pH, temperature, period of incubation was studied by using Czapek Dox as basal medium. Sucrose in the medium was substituted with pomegranate peel waste. The effect of metal ions of invertase was were also carried out. The results of the study revealed that the production of invertase was maximum on the 5th day of incubation at an optimum pH of 6.0 and optimum temperature of 300C. Purification of invertase was carried out using ammonium sulfate, dialysis followed by acetone precipitation. Protein content and thermal stability of the purified invertase was determined.
KEYWORDS: Invertase; Pomegranate peel; Cladosporium sp.
Download this article as:| Copy the following to cite this article: Uma C, Gopalakrishnan V. K. Utilization of pomegranate peel waste for the production and characterization of invertase using Cladosporium sp. Biosci Biotechnol Res Asia 2008;5(1) |
| Copy the following to cite this URL: Uma C, Gopalakrishnan V. K. Utilization of pomegranate peel waste for the production and characterization of invertase using Cladosporium sp. Biosci Biotechnol Res Asia 2008;5(1). Available from: https://www.biotech-asia.org/?p=6741 |
Introduction
The enzyme invertase (B-D-fructo furanside fructohydrolase, E.C.No:3.2.1.26) catalyses the hydrolysis of any compound with an unsubstituted b –D-fructofuranosyl residue and the prime substrate is sucrose.(George Boguslawski, 1983).
A wide range of microorganisms produce invertase and can thus, utilize sucrose as a nutrient. They hydrolyse sucrose to produce fructose and glucose in an equimolar mixture named inverted sugar. The inverted sugar is incorporated more easily in industrial preparations and has more added value than sucrose. (Chou and Jasovsky ,1993).
The high cost of invertase production process, together with the low process extraction yield and purification have been the limiting factors for the use of the enzymatic hydrolysis in the industrial process. Reduction in the production cost could make it a more efficient and reliable technique to be used industrially for the commercial production (Rodrigues et al., 2000).
Invertase is basically a yeast derived enzyme. But many microorganisms including fungi and bacteria, produce this enzyme. Invertase production by microorganisms such as Aureobasidium pullulans KFCC10525 (Kurkaka et al., 1996), Zymomonas mobilis (Park et al., 1991) and Clavibacter michiganensis (Baer et al., 1998) are known. (A.B.Dhake and M.B patil , 2005).
Invertase has gained importance in recent times due to its various biotechnological applications. Major applications of invertase includes confectionary, beverage, bakery and other pharmaceutical formulations for the preparation of invert sugar and high fructose syrup(HFS) from sugar (Gehlawat, 2001). It is one of the most widely used enzymes in food industry where fructose is preferred than sucrose especially in the preparation of jams and candies, because it is sweeter and does not crystallize easily (Cuitlahuac Aranda et al., 2006).
Material and Methods
Isolation of pure culture
The fungi isolated from the soil and single germinating spores were picked from the mixed culture. A pure culture was produced by repeated subculturing which was transferred to agar slant and subcultured fortnightly which was then stored at 4° C until use (Anjali Hans and Arun Dev
Sharma, 2005).
Selection of organism based on the utilization of sucrose
The organism present in the pure culture were identified using “Lactophenol cotton blue mounting method”, which was especially used for the identification of fungi .The organism used through out the experiment was Cladosporium species was selected and identified commonly using 2% glucose medium (Srinivasan et al., 1991 Nakamura et al.,1997 and Domsch KM et al., 1980 ).
Growth media and cell suspensions
Cladosporium species was maintained in the Czapek Dox medium as basal medium and sucrose was substituted with pomegranate peel waste in the fermentation media as the carbon source. (Marcin Skowronek et al., 2003).
Culture condition
The production medium was inoculated with a loop of culture from 24 hours old stock culture slants (Gogoi et al., 1998) and incubated at 30°C. After 3 days of incubation, invertase, biomass, pH and reducing sugar content of the medium were determined for 7 days with 24 hours interval.
(Anjali Hans and Arun Dev sharma., 2005).
Cell free extract preparation
The mycelial mass was collected by filtration after 3 days of incubation .The filterate was centrifuged at 10,000 rpm for 20 minutes at 4°C .The supernatant was used as the source of invertase enzyme mainly contains extracellular enzyme. The mycelial mass was washed several times with distilled water and used for dry weight determination. The cell growth (biomass) in the liquid culture was monitored by dry weight that was expressed as gl-1 of the microbial culture. (Dhake and Patil, 2005; Anjali Hans and Arun Dev
Sharma 2005 )
Optimization of the culture condition
The enzyme activity was measured by altering the individual the parameter such as pH , temperature and substrate concentration of the medium keeping the remaining parameter unaltered. (Peter. F. Stanbury et al.,1997).
Assay of enzyme activity
The determination of invertase activity was carried out by DNSA method.
The crude enzyme was purified using ammonium sulfate, dialysis and acetone. The thermal stability of the purified invertase was performed by incubating the purified sample in 0.01M acetate buffer of pH 6.0 at 50°C respectively.
Enzyme units
One unit of invertase (IU) was defined as the amount of enzyme which liberated / mg of product / minute /ml under the assay condition . (Anjali Hans and Arun Dev Sharma ,2005)
Results and Discussion
Invertase production was reported in Cladosporium sp.,(Dhake and Patil, 2005).The cultural parameters have a significant impact on both enzyme production and the activity. The effect of some cultural parameters like incubation time, pH, temperature and substrate feeding (Pomegranate peel waste) has been studied on invertase production by Cladosporium.
The organism was grown for a period of 7 days. The enzyme activity was measured at regular intervals of 24hrs at pH 4-7. The maximum invertase was produced after 5 days of inoculation at pH 6 Table 1. Similar observation was obtained by Anaclaudia etal.,2005 using Cladosporium cladosporioides cells and by B.k.Gogoi etal., 1998 using Saccharomycopsis fibuligera.
Table 1: Effect of pH in Invertase production by Cladosporium Sp., (Pomegranate Peel Waste), Invertase activity IU/ml.
| pH Incubation Period (Days) | ||||
| III | IV | V | VI | VII |
| 4 1.323(0.91) | 1.344(1.08) | 2.646(2.13) | 1.890(1.49) | 1.806(1.30) |
| 5 1.932(1.86) | 2.436(2.06) | 3.255(3.99) | 3.150(3.63) | 2.499(2.09) |
| 6 2.877(2.57) | 3.108(2.89) | 4.494(6.31) | 4.284(5.20) | 2.835(2.45) |
| 7 1.785(1.32) | 2.394(1.99) | 3.192(3.81) | 2.840(2.84) | 2.289(1.89) |
The data in parenthesis indicates mycelial biomass
Optimum temperature for invertase production by Cladosporium was found to be 30°C Table 2, on the same 5th day. Kazhurio Fukui et al., 1974 also reported the temperature of 40°C as optimum temperature for enzyme production by streptococcus mutants. This observation was also supported by Shaik Yazdani Basha et al., 1994 using Thermomyces lagnuginosus.
Table 2: Effect of temperature in invertase production by Cladosporium Sp., (Pomegranate Peel Waste), Invertase activity IU/ml.
| Temperature Incubation Period (Days) | |||||
| (°C) | III | IV | V | VI | VII |
| 30 | 4.6(3.4) | 7.7(5.6) | 9(6.8) | 6(4.8) | 5.9(4.3) |
| 40 | 12.3(9.7) | 14.1(10.6) | 17.5(13.6) | 12(9.4) | 10.5(7.9) |
| 50 | 4.1(2.9) | 6.8(5.1) | 8(5.8) | 5.5(3.8) | 4(3.0) |
| 60 | 2.2(1.8) | 6.3(5.2) | 7(5.3) | 3(2.3) | 2(1.8) |
The data in parenthesis indicates mycelial biomass
The enzyme production at different substrate concentration (Pomegranate peel waste) 2-8 gm was also done. The maximum enzyme production was found to be at substrate concentration of 4gm at 5th day of incubation in optimum culture medium (Table 3). the Km value of the enzyme obtained after 5 days of incubation is 1.4 (Fig. 1) which was supported by Gogoi et al., 1998.
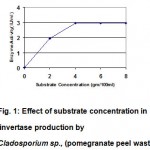 |
Figure 1: Effect of substrate concentration in invertase production by Cladosporium sp., (pomegranate peel waste).
|
Table 3: Effect of Substrate Concentration in Invertase production by Cladosporium Sp., (Pomegranate Peel Waste), Invertase activity IU/ml.
| Concentration Incubation Period (Days) | ||||
| gm/100ml III | IV | V |
|
VI
VII |
| 2 1.449(1.42) | 1.596(1.80) | 1.932(1.33) | 1.457(1.62) | 1.171(1.51) |
| 4 2.457(1.36) | 2.604(2.62) | 2.940(2.98) | 2.465(2.57)
2.179(2.19) |
|
| 6 2.457(1.53) | 2.604(2.86)
2.179(3.28) |
2.940(3.67)
|
2.465(3.47)
|
|
| 8 2.456(1.21) | 2.603(1.10)
.177(1.49) |
2.940(1.58)
|
2.464(1.31) 2
|
|
The data in parenthesis indicates mycelial biomass
Purification procedure resulted in a 2-fold increase in specific activity of the enzyme (Table 4). The thermal stability of partial purified invertase on exposure to different temperatures. The full activity was retained when the enzyme was exposed to 10 minutes at 50°C (Fig. 2). The result obtained was supported by Skowronek et al., 2003 by using the strain Cladosporium herbarum.
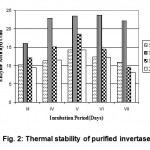 |
Figure 2: Thermal stability of purified invertase.
|
Table 4: Partial purification of invertase.
| Description
Specific |
Invertase activity
|
Total protein
|
|
| IU/ml | mg/ml | activity | |
| Crude extract | 0.924 | 0.421 | 2.196 |
| 30%Ammonium | 1.639 | 0.299 | 5.4 |
| sulphate saturation | |||
| Dialysed | 2.152 | 0.190 | 11.326 |
| 50%Acetone 2.659 0.156 17.04 | |||
The effect of metal ions on the activity of the enzyme was examined by incubated with various metal ions of concentration 0.01M at pH 6 and temperature 30°C for 10 minutes. The cations tested had a significant inhibitory effect on the hydrolysis by invertase. Zn was found to be a potent inhibitor of invertase (Fig. 3). The result obtained was supported by Gogoi et al ., 1998 by using the strain Saccharomycopsis fibuliger and concluded that no metal ions can activate the enzyme.
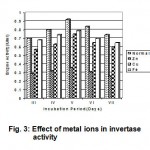 |
Figure 3: Effect of metal ions in invertase activity.
|
The present study concludes that the pomegranate peel waste can be more effectively used as a carbon source for the production of invertase using Cladosporium sp., under the optimized culture condition. Hence it can be substituted as a carbon source for the commercial production of invertase.
Acknowledgements
The authors thank our Management for providing lab support and constant encouragement.
References
- Anaclaudi Santana de Almeida, Luciares Costa de Aranjo., Andressa Mendes Costa., Cesar Augusto., Moraes de Abreu., Maria Alice Gomes de Lima and Maria de Los Angeles Perez Fernandez Palha. Sucrose hydrolysis catalysed by autoimmobilized invertase into intact cells of Cladosporium cladosporiodes. Electronic Journal of Biotechnology., 8: 717-785 (2005).
- Anjali Hans and Arun Dev Sharma. Production and partial characterization of invertase from Actinomycetes strains. Asian Journal of Microbiology and Biotechnology., 7: 205-208 (2005).
- Baer P., White A.R., and Neil C. Partial characterization of an extracellular b-Fructofuranosidase from Clavibacter michiganesis subspecies Sepedonieus. Canadian Journal of Microbiology., 44:
852- 865 (1998). - Chou C.C. and Jasovsky G.A. Advantages of Escorb T M precoats in liquid sugar production. International sugar Journal. 95: 425- 430 (1993).
- Cuitahuac Aranda., Armando robledo., Octavio Loera., Juan C. Contreras Esquivel., Raul Rodrigueq and Cristobal Neo Aguillar. Fungal invertase expression in soild state state fermentation. Food Technology Biotechnology., 44: 229-233 (2006).
- Dhake A.B., and Patil M.B. Production of acidic invertase by Pencillium perpurogenum and effect of substrate feeding. Asian Journal of Microbial Biotechnology Environmental Science., 7: 445-448 (2005).
- Domsch K.M., Gams W., and Traute Heidi Anderson. Compendium of soil fungi.
202- 210 (1980). - Gehlawat J.K. New technology for invert sugar and high fructose syrups from sugarcane. Indian Journal of chemical technology., 8: 28-32 (2001).
- George Boguslawski. A simplified method for measuring activity of b-D-Fructo-furanoside fructohydrolase (Invertase). Journal of applied Biochemistry., 5: 132-135 (1983)
- Gogoi B. K., Pillai K.R., Nigam J.N., and Bezbaruah R.L.. Extracelluar bAmylase and invertase from amylolytic yeast Saccharomycopsis fibuligera. Indian Journal of Microbiology. 38: 15-19 (1998)
- Kurkaka M.M., Onoue T and Komaki T. Effect of pH on transfructosylation and hydrolysis by b-Fructofuranoside from Aspergillus oryzae Applied Microbiology and Biotechnology., 45: 236-239 (1996).
- Nakamura T., Shitara A., Matsda S., Matsuo T., Suiko M. and Ohta K. Production, purification and properties of endoinulinase of Penicillium sp. TN88 that liberates inulotrios. Journal of fermentation bioengineering., 84: 313-318 (1997).
- Park J.K., Ro H.S. and Kim H.S. A new biosensor for determination of sucrose using an oxidoreductase of Zymomonas mobilis and Invertase. Biotechnology Bioengineering., 38: 217-223 (1991).
- Peter F. Stanbury., Allan Whitaker and Stephen J Hall. Principles of fermentation technology, Vol: II, 2ndEdition, Aditya Books (P) Ltd., New Delhi 110-116 (1997).
- Reddy S.M. and Ram Reddy S. Microbiology Laboratory manual.3rdEdition, Sri Padmavathi Publications, Hydrabad. (2005).
- Rodrigues J., Perez J.A., Ruiz T and Rodriguez L. Characterization of the Invertase from Pichia anomala. Biochemical Journal., 306: 235-239 (2000).
- Shaik Yazdani Basha and Peramachi Palanivelu Enhancement in the activity of an Invertase from the thermophilic fungus, Thermomyces launginosus by phospholipids. Indian Journal of Microbiology., 39: 217- 220 (1994).
- Srinivasan M.L, Laxman R.S and Deshpande M.V. Physiology and nutritional aspects of Actinomycetes: an overview. World Journal of Microbial Technology. 6: 167-169 (1991).

This work is licensed under a Creative Commons Attribution 4.0 International License.





