How to Cite | Publication History | PlumX Article Matrix
Seema Langer*, Anshu Samyal and Yahya Bakhtiyar
Department of Zoology, University of Jammu, Jammu - 180 006 India.
ABSTRACT: While studying the reproductive behavior during the different seasons of a year, the different developmental stages of oocytes in the ovary of M. dayanum (Hend.), a locally available freshwater prawn, have been recognized as: Stage 1: Oogonia, Stage 2: Previtellogenic oocytes, Stage 3: Vitellogenic oocytes, Stage 4: Mature oocytes. Gonadosomatic index (GSI) used estimating reproductive condition of the female prawn, increases concomitantly with maturation. A significant positive correlation exists between GSI and HSI (r=0.580, p<0.05). GSI showed a bimodal peak throughout the reproductive period. M. dayanum has high gonadal activity from March to May concomitant with highest average GSI in the month of April owing to the growth of vitellogenic oocytes and low GSI during immature phase.
KEYWORDS: Macrobrachium dayanum; Gonadosomatic; Hepatosomatic index
Download this article as:| Copy the following to cite this article: Langer S, Samyal A, Bakhtiyar Y. Studies on ovarian development of macrobrachium dayanum (Hend.) in relation to gonadosomatic index and hepatosomatic index. Biosci Biotechnol Res Asia 2008;5(1) |
| Copy the following to cite this URL: Langer S, Samyal A, Bakhtiyar Y. Studies on ovarian development of macrobrachium dayanum (Hend.) in relation to gonadosomatic index and hepatosomatic index. Biosci Biotechnol Res Asia 2008;5(1). Available from: https://www.biotech-asia.org/?p=6747 |
Introduction
Study of the reproductive biology, oocyte development and Gonadosomatic index is of prime importance for considering any species worth importance for culture and hatchery based production. Several workers have carried out studies on the reproductive biology of shrimps and other decapods crustaceans 1-4. Various penaeid and shrimp species viz. Penaeus japonicus5; Penaeus setiferus6; Penaeus idella7; Macrobrachium rosenbergii8 have been studied for their reproductive behavior. In crustaceans, the Gonadosomatic index is a gross indicator of good reproductive condition representing way to measure changes in size and weight of ovary during breeding period. Study of the reproductive biology in relation to Gonadosomatic index (GSI) was carried out in M. dayanum which is of significance with reference to brood stock management.
Material and Methods
Prawns were collected during morning hours (800-1100 hrs) from Gho-Manhasan stream because of easy access and availability in abundance throughout the year. Since stream is located at a short distance thus live specimens could be brought safely with less stress to the laboratory, where they were kept in plastic troughs. Mature healthy male and female individuals in the size range of 4cm to 5cm and above were segregated and kept in separate troughs.
Monthly collected samples of Macrobrachium dayanum were subjected to histological studies for a period of one year, beginning in May 2006 and concluding in April 2007. Parameters like; whole length, whole weight, gonad (ovary) weight, hepatopancreas (HP) weight, maturity stage, GSI and HSI were recorded for each prawn.
Gonadosomatic index (GSI ) was calculated by using the formula given below.
Permanent slides were prepared to study the different stages of sexual maturity by following standard methods. The slides so prepared were then studied under Nikon YS 100 microscope and photographed with the help of SDC-313 Camera.
Results
Different stages of ovarian development in m. dayanum, throughout the year
Immature ovaries are translucent mass in the early stage of development with dark black pigmentation and later on become orange in color which deepens with melanophore formation at the advanced stages of development. As it proceeds towards the egg formation it becomes greenish in color due to presence of mature ova. Structurally, the ovaries of M. dayanum resemble that of M. rosenbergii9 and Penaeus setiferus 6.
Depending upon the changes in nature and organization of the cytoplasm during different seasons, following four different stages of oocytes in the ovarian development of the fresh water prawn, M. dayanum have been recognized.
Developmental stages
Oogonia
In this stage, the oocyte is either oval or spherical measuring about 20 to 52.0 µ with centrally located round nucleus. Nucleolus is also observed either at the centre of nucleus or adhered to the nuclear membrane. Cytoplasm is homogeneous without any vacuoles or yolk globules (Fig. 1- 2).
In few oogonia of this stage binucleolate condition was also observed. During the developmental process it is the first differentiable oogonia and smallest in size. Oogonia are present either attached to germinal epithelium or lie freely in the ovocoel in the months of December to March and May to July.
Previtellogenic oocytes (Fig. 1and 2)
The oocytes of this stage measure from 45.0-195.0 µ. Ooplasm seems to be homogenous but in certain cells it is subjected to characteristic change with peripheral vacuolization i.e. vacuoles appear in the cytoplasm and are arranged concentrically. Nutritive cells arrange themselves along the periphery of these oocytes but distintive follicular layer (of nutritive cells) is seen in advanced stages. As the development proceeds this layer becomes prominent. Pre-vitellogenic oocytes are seen in different month’s viz. May to July and November – January.
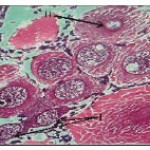 |
Figure 1
|
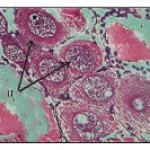 |
Figure 2
|
Vitellogenic oocytes (Fig. 3 and 4)
The oocytes of this stage are in the range of 230 to 400 µ. The cytoplasm though vacuolated exhibits granular nature. Small sized yolk granules make their appearance and spread centrifugally. There is considerable increase in the size of the nucleus and the cell. Nuclear membrane becomes indistinct. Nutritive cells form a distinct epithelium around the developing oocytes. Maximum numbers of stage III oocytes are mostly present in June-July and February-March.
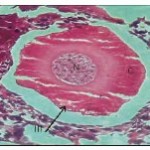 |
Figure 3
|
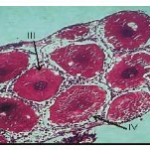 |
Figure 4
|
Mature oocytes. (Fig. 4)
Oocytes of this stage attain maximum size of about 650-950 µ. The yolk globules occupy the entire ooplasm. Nucleus is acentrically located. Yolk globules present at the periphery are spherical in shape and those located centrally are oval in shape and small sized. Each oocyte is surrounded by a clear demarcated layer of follicular epithelium. Between the follicular epithelium and the inner ooplasm develops a thin egg membrane. Maximum numbers of oocytes of this stage are present in the months of March-April and August to October.
Gonadosomatic index (GSI) and Hepatosomatic index (HSI)
Gonadosomatic index is a reliable indicator for understanding ovarian condition and estimation of reproductive condition of the female.
Perusal of table 1 showed that female GSI increases concomitantly with the maturation and presently observed change in its value from maximum in months of March (3.62 ± 1.12), April (3.90 ±1.02) and Aug. (2.45±0.31), Sept. (2.33±0.21), the two breeding seasons, when gonads are fully mature. In comparison to first phase of annual breeding period, the value of GSI during the IInd phase is comparatively low this may be due to the fact that they are left over brooders of first batch which do not get the chance to breed due to one or other reason.
Table 1: Seasonal variation in the Gonadosomatic index and Hepatosomatic index of Macrobrachium dayanum.
| Months | Avg. weight | Avg. weight of | Avg. weight | HSI | GSI |
| of animal | hepatopancreas | of gonads | |||
| May | 1.062 | 0.036 | 0.021 | 3.38+0.23de | 2.02+0.14cde |
| June | 1.059 | 0.032 | 0.023 | 3.02+0.21ef | 1.51+0.23e |
| July | 1.745 | 0.068 | 0.022 | 3.89+0.14d | 1.66+0.21de |
| August | 0.927 | 0.024 | 0.024 | 2.58+0.14f | 2.45+0.31cde |
| September | 1.21 | 0.026 | 0.022 | 2.13+0.16g | 2.33+0.21cde |
| October | 1.32 | 0.031 | 0.030 | 2.33+0.24fg | 2.29+0.16cde |
| November | 1.062 | 0.025 | 0.023 | 2.38+0.16g | 2.25+0.21cde |
| December | 1.022 | 0.090 | 0.022 | 8.80+0.25a | 2.15+0.36bcd |
| January | 1.005 | 0.056 | 0.026 | 5.17+0.24c | 2.67+0.33bcd |
| February | 0.745 | 0.049 | 0.021 | 6.57+0.25b | 2.95+1.15abc |
| March | 0.634 | 0.056 | 0.023 | 8.83+0.64a | 3.62+1.12ab |
| April | 0.634 | 0.039 | 0.023 | 6.62+0.99b | 3.90+1.02a |
Statistical analysis (Pearson’s correlation and Duncan’s ONE WAY ANOVA) reveal a significant positive correlation between GSI and HSI (r=0.580, P<0.05).
Discussion
Studies have revealed that M. dayanum is a biannual breeder: first phase of annual breeding occurs in the months of April-June and second phase of annual breeding lasts from August to September. A similar trend of biannual breeding in crustaceans is already on record 3,10,11,12,13,14.
Structurally, the ovaries of M. dayanum resemble that of M. rosenbergii (Chakravarty, 2003) and Penaeus setiferus6.
The ovarian wall of M. dayanum comprises of connective tissue lined internally by germinal epithelium. Both connective tissue and germinal epithelium at places extends inward to divide ovocoel into number of ovarioles. Contrary to this however, various workers1,7,15 have observed that ovarian wall consists of outer thin epithelium followed by layer of connective tissue and germinal epithelium.
The observation made on ovarian stages of M. dayanum are in contradiction to the studies on Paratelphusa hydrodromus16 and Cherax quadricarinatus5 have reported two phases: primary oocyte phase and secondary vitellogenic phase with bigger yolk globules.
M. dayanum has high gonadal activity from March to May concomitant with highest average GSI in the month of April owing to the growth of vitellogenic oocytes and low GSI during immature stages. Increase and decrease in GSI value concuss with histological condition of ovaries indicating annual changes in ovarian condition and it was also observed increase in average GSI corresponding with high gonadal activity in freshwater gobies (Rhinogobius brunneus)17, in support of our findings 18in female mosquito fish, Gambusia affinis and P. conchonius and C. gachua19 it has also been reported that GSI was found to increase during mature phases. Increase in GSI during vitellogenesis has also been observed in several teleost species species 20-23.
Significant, positive correlation between GSI and HSI is contradictory to many a scientists who observed inverse correlation between GSI and HSI 23-28.With maturation, GSI increases owing to the growth of oocytes and a rapid elevation in GSI first prior to spawning in female Atlantic Cod (Gadus morhua L.)29.
GSI is an indicative of reproductive activity and gonad condition but usually provides limited information of the internal changes occurring in the ovary, particularly with regard to gonadal development and composition34, 35 but still the morphological characteristics of each stage of oogenesis and ovarian development in relations to GSI are helpful in determining the pattern of gonadal development and energy use during vitellogenic process. Many authors have used GSI as a model for gonadal development in several teleosts32-37.
Acknowledgements
The authors are highly grateful to Head, Dept. of Zoology for providing necessary facilities and State DST (J and K Govt.) for providing financial assistance.
References
- Pillai, R.S., Studies on the Shrimp Caridina laevis (Heller) II. Reproductive system. J. Mar. Biol. Assoc., India, 2(1): 5774 (1960).
- Diwan, A.D. and Nagabhushnam, R., Reproductive cycle and histochemical changes in the gonad of freshwater crab, Barytelphus cunicularis (west wood). Indian J. Fish., 21: 164-176 (1974).
- Kailoo, U.C., Studies on systematics and reproductive biology of freshwater prawns of Jammu waters. M.Phil. thesis, Jammu University (1984).
- Abdu, U., Yehezkal, G. and Sagi, A., Oocyte development and polypeptide dynamics during ovarian maturation in the red claw crayfish, Cherax quadricarinatus. Invertebr. Repro. Dev., 37: 75-83 (2000).
- Hudinaga M., Reproduction, Development and Rearing of Penaeus japonicus Bate. Jpn. J. Zool., 10: 305-393 (1942).
- King, E.J., A study of the reproductive organs of the common shrimp, Penaeus setiferus. Biol. Bull. Woods Hole, 94: 244-262 (1948).
- Parmeswaran, R.. On the female reproductive system of Palaemon idella. J. Zool. Soc. India, 5: 227-234 (1953).
- Karplus, I. and Hulata, G., Social control of growth in Macrobrachium rosenbergii V, the effect of unilateral eyestalk ablation on jumpus and lazards. Aquaculture, 138:
1-4 (1995). - Chakravarty, M.S., Studies on the oocyte stages of the Prawn, Macrobrachium rosenbergii De Man. J. Aqua. Biol., 18(2): 101-106 (2003).
- Subramanyam, C.B., A note on the reproductive cycle of the prawn Penaeus indicus of Madras coast. Curr. Sci., 32:
165-166 (1963). - Ryan, E.P., A study of the reproductive biology of the hoale crab, Portunus sanguinoleutus (Herbst.) Ph. D. Thesis, Hawai University. (1965).
- Jegla, T.C.. Reproductive and moulting cycles in cave crayfish. Biol. Bull., 130(3): 345-358 (1966).
- Kulkarni, M.Y., Studies on the biology of
the freshwater prawn, Macrobrachium kistensis. Ph.D. Thesis, Marathwada Univ., Aurangabad. (1992). - Malik, N., Studies on female Reproductive system of Macrobrachium dayanum (Henderson). M.FSc. Dissertation Submitted to Directorate of distance education, University of Jammu. (2006).
- Patwardhan, S.S., The Indian Zoological memoirs and Indian animal types 6. Palaemon (The Indian River Prawn) 2nd ed. XIV + 102 + XVIII, result. Zoological survey of India, Culcutta. (1937).
- John, P.A. and Nair, K.K., Hormones functional in the reproductive activities of Crustacea. Symposium on comparative Endocrinology 36: 109-113 (1968).
- Katoh, M., Seasonal variation in gonadal activity of females among four species of freshwater gobies in the Rhinogobius brunneus species complex in Okinawa Japan. Ichthylogical Research, 43(2):
169-174 (1996). - Koya, Y., Itazu, T. and Inoue, M., Annual reproductive cycle based on histological changes in the ovary of the female mosquitofish, Gambusia affinis, in Central Japan. Ichthylogical Research, 45(3):
241-248 (1998). - Raina, S. Seasonal variations in biochemical composition of ovary, liver and muscles and histology and histochemistry of ovary in Channa gachua (Ham.) and Puntius conchonius (Ham.). Ph. D. thesis, University of Jammu (1999).
- Egami, M., Effect of estrogen and androgen on the weight and structure of the liver of the fish, Oryzias catipes. Annot. Zool. Japan, 28: 79-85. (1955).
- Aida, K., Hirose, K., Yokote, M. and Hibiya, T., Physiological studies on gonadal maturation of fishes-II Histological changes in the liver cells of ayu following gonadal, maturation and estrogen administration. Bull. Jap. Soc. Sci. Fish., 39: 107-115 (1973).
- Korsgaard, B. and Peterson, I.. Vitellogensis, lipid and carbohydrate metabolism during vitellogenesis and pregnancy and after hormonal induction in the blenny Zoarces viviparous (L.) Comp. Biochem. Physiol., 63B: 245-251 (1979).
- Boheman, Ch.G.van, Lambert, J.G.D. and Peute, J., Annual changes in plasma and liver in relation to vitellogenesis in the female rainbow brout, Salmo giardneri. Gen. comp. Enocrinol., 44: 94-107 (1981).
- Castille, F.L.and Lawrence, L.A. Relationship between maturation and biochemical composition of the Gonads and Digestive glands of the shrimps Penaeus aztecus Ives and Penaeus setiferus (L.) J. Crust. Biol., 9: 202-211 (1989).
- Palacios, E., Ibarra, A.M. and Racolta, I.S.,. Tissue biochemical composition in relation to multiple spawning in wild and pond reared Penaeus vannamei broodstock. Aquaculture, 185: 353-371 (2000).
- Millamena, O.M. and Pascual, F.P., Tissue lipid content and amino acid composition of Penaeus monodon Fabricius broodstock from the wild. J. World Aquac. Soc. 21:
116-121 (1990). - Cavalli, R.O., Tamlin Lavens, P. and Sorgeloor P., Variations in lipid classes and fatty acid content in tissue of wild Macrobrachium rosenbergii (de Man) females during maturation. Aquaculture, 193 (3-4): 311-324 (2001).
- Rodriguez-Gonzalez, H. Effect of protein and lipids content in the gonad development of females of the freshwater Australian crayfish, Cherax quadricarinatus (Con Martens). Master thesis, Centho de Investigaciones Bio-ogicas del Noroeste, La Paz, B.C.S.,Mexico, 86 (2001).
- Dahle, R., Taranger, Karlsen, G.L., Kjesbu, O.S. and Norberg, B., Gonadal development and associated changes in liver size and sexual steroids during the reproductive cycle of captive male and female Atlantic Cod (Gadus morhua L.) Comp. Biochem. And Physiol., 136 A: 641-653 (2003).
- Grant, A. and Tyler, P.A.. The analysis of data in studied of invertebrate reproduction: Introduction and statistical analysis of gonad indices and maturation indices. Int. J. Invertebr. Reprod., 6: 259-269 (1983).
- McRae, T.G. and Mitchell, B.D.. Studies on ovarian development in the yabby, Cherax albidus Clark. In: Fielder, D.R. Richardson, A.M.M. (Eds.), Freshwater Crayfish, Vol., 10, 10thInt. Sym. Of Astacology, Louisiana State Univ., Baton Rouge, LA, USA, 521-531 (1995).
- De Vlaming, V.L.. Environmental control of teleost reproductive cycle: a brief review: J. Fish. Biol. 4: 131-140 (1972).
- De Vlaming, V.L., Wiley, H.S., Delahunty, G. and Wallace, R.A. Goldfish (Carassius auratus) vitellogenin Induction Isolation, properties and relationships to yolk proteins. Comp. Biochem. Physiol., 67B: 613-623 (1980).
- Greelay, M.S. and MacGregor, J.R., Annual and semilunar tidal cycles. Copia, 8:
711-718 (1983). - Hay, D.E., Qutram, D.N., Mckeown, B.A and Hurlbust. Ovarian development and oocyte diameter as Maturation Criteria in Pacific Herring (Clupea harengus Pallasi). Can. J. Fish. Aquat. Sci., 44: 1496-1502 (1987).
- Treasurer, J.W., The annual reproductive cycle of Pike, Esox lucius (L.) in two Scottish lake: J. Fish., 36: 29-46 (1990).
- Garray, E. and Luna, S.R.. Gonadal development and spawning of female ocean white fish Caulatilus princes (Pisces; Branchiotegidae) in the Bay of La Paz, B.C.S. Mexico. J. Fish. Biol., 44: 583-566 (1994).

This work is licensed under a Creative Commons Attribution 4.0 International License.





