How to Cite | Publication History | PlumX Article Matrix
Effect of Spray Application of 2, 4-D on Anatomical Characters of Hibiscus Cannabinus Linn.
Sanjay I. Kamble
Department of Botany and Coordinator, Department of Seed Technology, Phulsing Naik Mahavidyalaya, Pusad, Dist. Yavatmal India.
ABSTRACT: In present study, the herbicidal activities of 2,4-D on Hibiscus cannabinus Linn. has been studied. The anatomical response might produce some light on the manner by which this compound affected on plant. The plants were sprayed with aqueous solution of different concentrations of herbicide from 100 to 10,000 ppm. The herbicide induced some anatomical changes like proliferation of cambium and phloem in the stem to form large masses of meristematic cells and due to the proliferation of cortical and pith cells after treatment became crushed. In the leaves, desiccation of cells, proliferation of cambium in the midrib region and distortion of vascular elements was common feature observed of 2,4-D treatment.
KEYWORDS: Herbicide; 2,4-D; Spray application; Anatomical characters; Hibiscus cannabinus Linn.
Download this article as:| Copy the following to cite this article: Kamble S. I. Effect of Spray Application of 2, 4-D on Anatomical Characters of Hibiscus Cannabinus Linn.. Biosci Biotechnol Res Asia 2008;5(1) |
| Copy the following to cite this URL: Kamble S. I. Effect of Spray Application of 2, 4-D on Anatomical Characters of Hibiscus Cannabinus Linn.. Biosci Biotechnol Res Asia 2008;5(1). Available from: https://www.biotech-asia.org/?p=6924 |
Materials and Methods
Plants of Hibiscus cannabinus Linn. were raised from seeds collected from naturally growing plants of different places in Nagpur and its environs. They were allowed to grow till they attained the flowering and at this stage plants were plants were sprayed with different concentrations of 2,4-D.
The aqueous solution of herbicide ranging from 100 to 5000 ppm was prepared. Ten pots for each concentrations (100 to 2000 ppm) containing 2 to 3 plants were sprayed. If 2000 ppm was found higher; the lower concentrations were tried to determined lethal dose. Asppe- poly sprayer of one litter capacity did spraying. A small quantity of sodium lauryl sulphasate as a surfactant added in the herbicide solution. The Spraying was started in month of October 1996 and same experiments were repeated next year also. Spraying was done twice in an hour to make it more effective in the evening hours, when the wind was slow and temperature comparatively lower than that of the day. This help in less evaporation and more absorption of herbicide solution by the leaves. To avoid contamination of different concentrations of herbicide, cardboard was used at the time of spraying application. Six pots were sprayed with water used as control. Field trials were conducted on naturally growing plants in randomly designed plots of size approximately 3 O3 feet’s.
The fresh and dry weights of shoots and roots of control as well as treated plants were taken to determined desiccation of plants. Morphological changes were observed daily till the death of plants.
To study the anatomical changes induced by herbicide, plant parts like root, stem, petiole and leaf of the treated as well as control plants were fixed in F: A: A (Formalin: Acetic acid : Alcohol ) solution for 24 hours and stored at 70% alcohol. The plant material was embedded in paraffin wax following customary method (Sass, 1951). Section was cut at 6 to 9 microns. They were then stained according to crystal violet – erythrosine schedule and mounted in D.P.X. Microphotograph of various sections of both control and treated plants were taken.
Results
Control
The control stem showed defined single layered epidermis followed by ground tissue. It was divided in to three zones; the outer zone was chlorenchymatous which was made up of 2 to 3 layers of parenchymatous cells containing chloroplast. This zone was followed by 2 to 3 layers collenchymas were immediately followed by 3 to 4 layers of parenchymatous cells. Endodermis and pericycle were distinct. Vascular tissues were arranged in a ring and enclose large parenchymatous pith (Fig. 1).
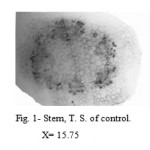 |
Figure 1: Stem, T. S. of control.
|
The root showed secondary growth and consisted of an outer epidermis, parenchymatous cortex followed by ring of secondary phloem and xylem. The distinct metaxylam was observed with a large vessels (Fig. 2).
 |
Figure 2: Root, T. S. of control.
|
The outer layer of petiole was made up of epidermis. Hypodermis was below the epidermis. Vascular bundles consisted of xylem and phloem (Fig. 3).
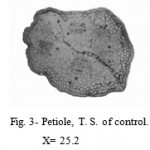 |
Figure 3: Petiole, T. S. of control.
|
The leaf was composed of three types of tissue system, the epidermal, mesophyll and vascular. The lamina has upper and lower epidermis. The mesophyll comprises upper palisade parenchyma and lower spongy parenchyma. The midrib comprises of parenchymatous tissue embedding vascular elements (Fig. 4).
 |
Figure 4: Leaf, T. S. of control.
|
2,4-D
2,4-D induced anatomical changes in stem, root, petiole and leaf of Hibiscus cannabinus Linn. The stem at 100 and 200 ppm showed an abnormal meristematic activity of cambium. Owing to periclinal and anticlinal divisions of cambium, the phloem was pushed outwards and crushed; whereas at 300 and 400 ppm the phloem and rays cells proliferated which results in the formation of meristematic masses. These masses of meristematic cells encroach centrifugally upon the fundamental parenchymatous cortex, disrupting it and finally ruptured the epidermis at many places (Fig. 5).
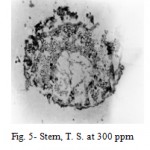 |
Figure 5: Stem, T. S. at 300 ppm.
|
The root at 100 and 200 ppm showed proliferated masses in the cortical region developed from the cells of cambium and phloem. However at 300 and 400 ppm these proliferated masses grow in cortex, crushing its cell and forming lacunae. Subsequently, the cortex and epidermis were disorganized and lost their identity (Fig. 6).
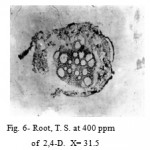 |
Figure 6: Root, T. S. at 400 ppm
|
The petiole at 100 and 200 ppm showed proliferation of cambium and phloem parenchyma calls. Owing to formation of meristematic masses, the pressure exerted on the cortical cells, lead to disorganization of cortex and destruction of epidermis (Fig. 7).
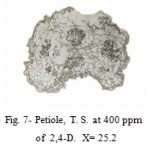 |
Figure 7: Petiole, T. S. at 400 ppm.
|
The leaves were severely injured at all concentrations of herbicide. At 100 and 200 ppm leaf showed desiccation of tissues and it lost cellular identity of epidermis. In midrib the parenchymatous cells of phloem were destroyed at 300 and 400 ppm and the mesophylls of the lamina also lost their identity (Fig. 8).
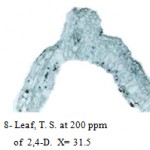 |
Figure 8: Leaf, T. S. at 200 ppm.
|
Discussion
This herbicide induced some anatomical changes in stem, root, leaves and petiole at all concentrations. Stem of the plants showed proliferation of phloem forming meristematic masses and disorganization of cortical cells were observed. In addition to it ruptured epidermis was observed at all concentrations and pith cells were destroyed and lost their identity. Rubin and Gritsaenta (1968) on Amaranths retroflexux and Chenopodium album reported the proliferation of cambium tissue due to application of 2,4-D. Similar results have been reported by Dnyansager and Khosla (1968) on Cassia tora, Kiepal (1970) on Sinapis arvensis, Bequest (1971) on Myriophyllum spicatum, Hadke, (1980) on Psoralea corylifolia and Euphorbia geniculata, Deshmukh (1981) on Cassia occidentalis, Corchorus olitorious, and Lagasca molis, Srinivasu (1986) on Parthenium hysterophorus, Bobde (1993) on Crotalaria juncea, Jain (1993) on Chenopodium album and Gopal (1993) on Medicago sativa.
In the leaves disorganization of mesophylls and destruction of phloem cells in midrib was observed due to application of 2,4-D. Tukey et.al. (1945) on bind weed reported the plasmolysis of mesophyll cells and lost their contents. Loustalot and Muzik (1953) on Stizolobium decringianum reported destruction in mesophyll cells. Similarly, Scifres and Mc Carty (1968) on Vernonia spp., Bakale (1972) on Alternanthera polygonoides and Cressa cretica, Walsh et.al. (1972) on Rhizophora maingle, White and Hemphill (1972) on Tobacco. Bakale and Dnyansagar (1977) on Xanthium strumarium, Kolhe (1979) on Tephrosia hamiltonii, Solanum surrattense, and Celosia argentea, Hadke (1980) on Psoralea corylifolia and Euphorbia geniculata. Deshmukh (1981) on Cassia occidentalis, Corchorus olitorious and Lagasca mollis, Srinivasu (1986) on Parthenium hysterophorus, Bobde (1993) on Crotalaria juncea, Jain (1993) on Chenopodium album and Gopal (1993) on Medicago sativa reported disorganization of mesophyll and destruction of phloem in mid vein.
In root 2,4-D induced certain anatomical changes such as formation of huge masses of meristematic cells developed from cambium and phloem, destruction of cortical and pith cells were observed at all concentrations of herbicide. Similar results were reported by Tukey et.al. (1945) on bind weed, Leroux (1957) on Nigella damascena, Kilpatrick et.al. (1963) on Trifolium spp., Audus (1964) on some weeds, Calhan and Engel (1965) on Agrostis fenuis, Kolhe (1979) on Tephrosia hamiltonii, Solanum surrattense, and Celosia argentea, Hadke (1980) on Psoralea corylifolia and Euphorbia geniculata. Deshmukh (1981) on Cassia occidentalis, Corchorus olitorious and Lagasca mollis, Srinivasu (1986) on Parthenium hysterophorus, Bobde (1993) on Crotalaria juncea, Jain (1993) on Chenopodium album and Gopal (1993) on Medicago sativa due to application of 2,4-D.
The petiole showed Proliferation of cambium, destruction of phloem cells, disorganization of cortex and endodermis in the present study. Many workers like Bradley et.al. (1968) on Apricot spp., Dnyansagar and Khosla (1968) on Cassia tora, Kolhe (1979) on Tephrosia hamiltonii, Solanum surrattense, and Celosia argentea, Hadke (1980) on Psoralea corylifolia and Euphorbia geniculata. Deshmukh (1981) on Cassia occidentalis, Corchorus olitorious and Lagasca mollis, Srinivasu (1986) on Parthenium hysterophorus, Bobde (1993) on Crotalaria juncea, Jain (1993) on Chenopodium album and Gopal (1993) on Medicago sativa reported similar results.
In the presents study, death of weed probably could occur due to proliferation and disorganizations of mesophyll cells in leaves. Distortion of plant apices and splitting of outer tissues of root and heavy meristematic activity was observed due to application of 2,4-D.
References
- Audus, L.S. 1964. Studies on pH relationship of root growth and its inhibition by 2,4-D and . coumarin. New Phytol. 48: 97-144.
- Bakale, V.L. 1972. Effect of folliar application of weedicide on Alternanthera polygonoides var.eracta Linn. The Botanique. 10(1-4): 89-101.
- Bakale, V.L. and Dnyansagar, V.R. 1977. Effect of weedicide on Xanthium strumarium Linn. J. Indian. Bot. Soc. 56(2): 116-120.
- Brequest, E.T. 1971. Ecological and Morphological effects on Myriophyllum spicatum Linn. In a Tennessee valley reservoir. Diss. Abstr. Internet B. 32(116): 137.
- Bobde, S.N. 1993. Comparative effects of herbicides on Crotalaria juncea Linn. Ph.D. Thesis, Nagpur Univ., Nagpur.
- Bradley, M.V., Crane, J.C. and Marie, N. 1968. Some histological effects 2,4,5-T applied to mature Apricot leaves. Bot. Gaz. 129: 231- 238.
- Calhan, L.H. and Engel 1965. Tissue abnormalities induced in root of Agrostis fenuis by phenoxy alkyl carboxylic acid herbicide. Weeds. 13: 336-338.
- Deshmukh, V.R. 1981. Effect of weedicides on cytomorphology of weeds. Ph.D. Thesis, Nagpur Univ., Nagpur.
- Dnyansager, V.R. and Khosla, S.N. 1968. Effects of 2,4-D on anatomical characters of some weeds. Proc. Ind. Acad. Sci. 703: 287-294.
- Gopal, K.R. 1993. Herbicidal effects on cytomorphology of weeds Medicago sativa. . Ph.D. Thesis, Nagpur Univ., Nagpur.
- Hadke, S.M. 1980. Effect of herbicide on cytomorphology of weeds Psoralea corylifolia and Euphorbia geniculata. Ph.D. Thesis, Nagpur Univ., Nagpur.
- Jain, S.B. 1993. Cytomorphological effects of weedicides on weed Chenopodium album. Ph.D. Thesis, Nagpur Univ., Nagpur.
- Kiepal, Z. 1970. Pathological anatomy of Sinapis arvensis Linn. Stem histological, anatomical changes caused by herbicides in the auxin type. Acta Agrobotanica. 23: 73-84.
- Kilpatrick, R.A., Chen, A.E. and Rodriguez, Z. 1963. Root symptoms and anatomical changes in Trifolium spp. by 2,4-D. Pl. Diss. Reptr. 47: 497-501.
- Kolhe, R.R. 1979. Effect of herbicides on the cytomorphology of farm weeds. Ph.D. Thesis, Nagpur Univ., Nagpur.
- Leroux, R. 1957. Research on morphological and histological modification on Nigella damascena Linn. Caused by 2,4-D. Rev. Gen. Bot. 64: 299-306.
- Loustalot, A.J. and Muzik, T.J. 1953. Effect of 2,4-D on apparent photosynthesis and developmental morphology of Stizolobium decringianum. Bot. Gaz. 115: 56.
- Rubin, S.S. and Gritsaenta, A.M. 1968. The effect of 2,4-D on the structure of plants. Bot. Gaz. 53: 377-378.
- Scifres, C.J. and Mc Carty, M.K. 1968. Reaction of western ironweed (Vernonia spp.,) leaf tissue to picloram. Weed Science. 34: 347-349.
- Srinivasu, T. 1986. Effect of weedicide on Parthenium hysterophorus Linn. Ph.D. Thesis, Nagpur Univ., Nagpur.
- Tukey, H.B., Hamner, C.L. and Imhofe, B. 1945. Histological changes in bind weed sowthistle concentrations. Bot. Gaz. 107: 62-73.
- Walsh, G.E., Cook, G.H. and Barvett, R. 1972. Rhizophora maingle Linn. Effect and translocation of 2,4-D and piloram. Abstr. Meet. Weed Sci. Soc. America. Pp.11.
- White, J.A. and Hemphill, D. 1972. An ultra structure study of 2,4-D on Tobacco leaves. Weed Science. 20: 478-481.

This work is licensed under a Creative Commons Attribution 4.0 International License.





