How to Cite | Publication History | PlumX Article Matrix
N. Adib1 and Et Al2,
1Food and Drug Laboratory Research Center, Food and Drug Control Labs, Ministry of Heath, Tehran Iran
2Department of Medicinal Chemistry, School of Pharmacy, Shaheed Beheshti Medical University, Tehran Iran.
Corresponding Author E-mail: nadib@fdo.ir
ABSTRACT: A simple, rapid and sensitive isocratic reversed-phase HPLC method with UV detection is described based on external-standard calibration for determination of ciprofloxacin in plasma samples during bioequivalence studies. Both internal and external procedures were evaluated and the external-standard method demonstrated a high validity based on ICH criteria. After protein precipitation with acetonitrile and dichloromethane, chromatographic analysis of ciprofloxacin in plasma was carried out on a μ-bondapack C18 column using acetonitrile:0.005 M tetrabutylammonium bromide (10:90) mixture, pH 2, as mobile phase. Quantitative determination was performed by ultraviolet detector at 278 nm. The method was specific and validated with a limit of detection of 20 ng/ml and limit of quantitation of 50ng/ml. The intra- and inter-day coefficients of variation were in the range of 1.51-4.48% and 4.02-7.3%, respectively. The recovery of method was 94.27±1.91%. The method was applied to a bioequivalence study of two formulations containing 500 mg ciprofloxacin.
KEYWORDS:
Ciprofloxacin; Human plasma; HPLC; Bioequivalence.
Download this article as:| Copy the following to cite this article: Adib N, Al E. A New HPLC Method for Determination of Ciprofloxacin in Human Plasma and its Application in Bioequivalence Studies. Biosci Biotechnol Res Asia 2008;5(2) |
| Copy the following to cite this URL: Adib N, Al E. A New HPLC Method for Determination of Ciprofloxacin in Human Plasma and its Application in Bioequivalence Studies. Biosci Biotechnol Res Asia 2008;5(2) Available from: https://www.biotech-asia.org/?p=7160 |
Introduction
Ciprofloxacin is a broad-spectrum fluoroquinolone antibacterial agent that is active against a wide range of aerobic gram-positive and gram-negative organisms. It acts by inhibiting the bacterial enzyme DNA gyrase, which is needed for DNA synthesis. Ciprofloxacin is used in a wide variety of situations such as lower respiratory tract infections, complicated urinary tract infections, gastrointestinal as well as skin and soft tissue infections 1-6. In comparison with other non-fluorinated quinolones, ciprofloxacin has a good oral absorption and a better bioavailability. It has an approximate bioavailability of 70%. Maximum plasma concentrations (0.8 to 3.9 mg/ml) are achieved within 1 to 2 hours after oral administration of single 500 mg dose. Food has been shown to prolong the time to reach maximum concentration. The drug has a large volume of distribution (2.1 to 5 l/kg) and is concentrated in many body tissues and fluids, including bile, kidney, liver, gallbladder, prostate and lung tissues. Ciprofloxacin is metabolized in the liver to at least four metabolites three of them have some antimicrobial activity. In oral administration, ciprofloxacin is largely excreted unmetabolized in the urine (25-30%) and feces (50%), although small amounts of metabolites (22%) have been detected in urine. The elimination half-life of ciprofloxacin is about 3-6 hours.
Several HPLC methods have been reported for analysis of ciprofloxacin in biological fluids. Some of these methods have employed UV detection 7-10, while the others have employed fluorescence detection 11-19. Some of the reported methods used expensive sample extractions by means of switching devices or ion-pairing liquid chromatography 20, 21, time and material consuming extraction methods such as liquid-liquid extraction 22-25 or derivatization methods. 26
The aim of this study was to develop a simple, rapid, sensitive and reliable HPLC method with UV detection for quantitative determination of ciprofloxacin in human plasma samples. The method was validated to provide enough selectivity, sensitivity and reliability in pharmacokinetic and bioequivalence studies.
Materials and Methods
Chemicals
Acetonitrile (HPLC grade), phosphoric acid (analytical grade), methylene chloride (analytical grade) and tetrabutylammonium bromide were purchased from Merck. Ciprofloxacin was USP reference standard.
Sample preparation
To a 0.5 ml aliquot of plasma 20 µl phosphoric acid 85% was added and vortexed for 20 s. After adding acetonitrile (2ml), the mixture was vortexed for 30 s to achieve deproteinization, and centrifuged at 4000 rpm for 10 min at 20 0 C. The protein precipitate was discarded, and 1.5 ml of the supernatant was added to 4 ml methylene chloride in a conical shape centrifuge tube. The tube was vortexed for 20 s and centrifuged for 5 min at 4000 rpm. In this step the methylene chloride extracted the acetonitrile away from the ciprofloxacin containing aqueous layer. The organic bottom layer was then discarded. Following a brief centrifugation 20 µl of the upper aqueous layer was injected. Between injections the syringe and loop were rinsed twice with 0.85% sodium chloride.
Instrument and chromatographic conditions
Analyses were performed on a Knauer model K-1001 pump equipped with a Knauer K-266 UV detector. Chromatography was performed at room temperature on a µ-bondapack C18 column (5 µm particle size, 25 cm × 4.6 mm I.D.). The mobile phase consisted of acetonitrile- 0.005 M tetrabutylammonium bromide (10:90) mixture, pH 2 which was adjusted using phosphoric acid. The flow rate was set at 1 ml/min and the eluent monitored with UV detector set at 278 nm.
Validation
Validation was accomplished through determination of specificity, recovery, linearity, quantitation limit, precision and accuracy. Specificity was investigated by analyzing six drug-free plasma samples for interference of endogenous compounds. Recovery was determined by comparing the response of pre-treated quality control plasma samples (0.05, 0.5, 2 µg/ml) with the response of identical samples standards prepared in the mobile phase which did not undergo sample pre-treatment.
Ciprofloxacin standard curve was obtained through the analysis of ciprofloxacin standard plasma samples and plotting the peak area versus the corresponding ciprofloxacin concentrations (0.05, 0.1, 0.5, 1, 2, 3, 5 µg/ml). Linearity of the standard curve was evaluated using least-squares linear regression analysis. Quantitation limit was defined as the lowest ciprofloxacin concentration that could be determined with mean value deviation and coefficient of variation less than 20%, using five plasma samples. The intra and inter-day precision (CV %) of the assay procedure were determined by repeated analysis of quality control plasma samples (0.05, 0.5 and 2 µg/ml) at the same day and different days.
Application
The validated method was successfully applied in a bioequivalence study of two dosage form tablets containing 500 mg ciprofloxacin (test and reference). The blood collecting times were 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, and 24 h after oral administration of 500 mg ciprofloxacin reference and test. The plasma samples were analyzed by the mentioned method.
Result and Discussion
The proposed method is suitable for ciprofloxacin quantification in plasma samples. It showed specificity, since no interfering peaks from endogenous components of plasma were observed. Typical chromatograms are shown in figure 1. The method was linear over the range of 0.05- 5 µg/ml and the calibration curve could be described by the equation y=69.51x-227.59 (r2=0.999) .
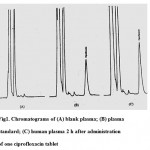 |
Figure 1: Chromatograms of (A) blank plasma; (B) plasma standard; (C) human plasma 2 h after administration of one ciprofloxacin tablet.
|
The limit of quantitation (LOQ) was 0.05 µg/ml and limit of detection (LOD) was 0.02 µg/ml. The intra and inter-day precision (CV %) of the quality control samples were 1.51- 4.48 and 4.02-7.3% respectively with Pvalue of 0.05% . (table1, table2)
Table 1
Intra-day precision (n=3)
| C nominal, µg/ml | 0.05 | 0.5 | 2 |
| Mean Cfound, µg/ml | 0.051 | 0.477 | 2.093 |
| STD | 2.47 | 7.25 | 52.8 |
| CV% | 4.48 | 1.51 | 2.53 |
Table 2: Inter-day precision (n=3)
| C nominal, µg/ml | 0.05 | 0.5 | 2 |
| Mean Cfound, µg/ml | 0.047 | 0.494 | 2.011 |
| STD | 3.44 | 19.88 | 105.89 |
| CV% | 7.3 | 4.02 | 5.26 |
This HPLC method is reliable, reproducible, sensitive and suitable for the analysis of ciprofloxacin in plasma and is applicable to pharmacokinetic studies. The mean concentration profiles of healthy human volunteers after receiving one tablet 500 mg ciprofloxacin (test and reference products) are given in fig 2.
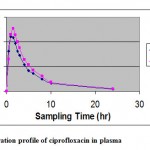 |
Figure 2: Concentration profile of ciprofloxacin in plasma.
|
Statistical comparison of the AUC0-24 and Cmax clearly indicated no significant difference between test and reference of 500 mg tablets, in any of the calculated pharmacokinetic parameters p>0.05. And these values are entirely within the bioequivalence acceptance range of 80-125% (using log transformed data).
Several HPLC methods have been developed for determination of ciprofloxacin in plasma, but these are too complicated and time-consuming for application in bioequivalence assays. One of the challenging aspects of method development in quantitative analysis of a compound is the simplicity of the method. The simpler method the better it could be conducted by different operators and in different labs. However other parameters of a quantitative method such as accuracy and precision demand more complex processes. One of the routine attempts for the improvement of accuracy and precision of a method specially when it comes to the analysis of plasma samples is the utilization of an internal standard which compensates errors which are originated from extraction processes as well as some of the operator’s errors such as volume of injection. In the meantime, using an internal standard makes the method more complex and more time consuming. Therefore if the use of internal standard could be omitted from the analysis process without losing accuracy and precision, the method would have the advantage of being simpler and faster.
In the present study we were able to develop a method of quantitative analysis for ciprofloxacin in plasma which dose not need an internal standard. Ciprofloxacin has three basic sites in its structure and therefore it could be easily protonated and become ionized in acidic condition. Therefore the method was designed based on a simple protein precipitation using acetonitrile. Since the method dose not involves any other extraction step, the recovery percent was found %94.27 ± 11.91 for the concentration range of 0.05 to 5 µg/ml. Both automatic injection and manual injection gave satisfactory results. However as it could be anticipated, automatic injection is the recommended injection method for this method. In order to intercept any mechanical problem in the auto injector which may cause error in the results, it is recommended to inject at least one QC (quality control) sample after every ten injections.
With respect to validation parameters this HPLC method is reliable, reproducible, sensitive and suitable for the analysis of ciprofloxacin in plasma and is applicable in pharmacokinetic studies.
Acknowledgment
This work was made possible by the special efforts of Amin Pharmaceutical Company.
References
- Muytjens H.L., Van J., Myers C.M. and Blumer J.L., J. Chromatography, 422, 153-164 (1987).
- Wise R., Andews J.M. and Edwards L.J., Antimicrob. Agents Chemother., 23, 559-564 (1983).
- Roy C., Foz A., Segusa C., Tirado M., and Tesvel D., Infection, 11, 326-328 (1983).
- Chin N.X. and Neu H.C., Antimicrob. Agents Chemother., 25, 319-326 (1984).
- Wolfson J.S. and D.C. Hooper, Antimicrob. Agents Chemother., 28, 581 (1985).
- Smith, J.M., Br. J. Pharm. Pract., 10, 18-23 (1988).
- Jehl F., Gallion C., Debs J., Brogard J.M., Monteil H. and Minck R., J. Chromatogr., 339, 347 (1985).
- Weber A., Chaffin D., Smith A. and Opheim K.E., Antimicrob. Agents Chemother., 27, 531 (1985).
- Greenveld A.J.N. and Brouwers J.R.B., Pharm Weekble, 8, 79 (1986).
- Maya M.T., Goncalves N. J., Silva N. B. and Morais J. A., J. Chromatog. B Biomed. Sci. Appl., 755, 305-9 (2001).
- Nix D.E., De vito J.M. and Schentag J.J., Clin. Chem., 31, 684 (1985).
- Boner K., J. Clin. Chem. Biochem., 24

This work is licensed under a Creative Commons Attribution 4.0 International License.





