How to Cite | Publication History | PlumX Article Matrix
Ajay D. Kshirsagar and Purnima Ashok*
Department of Pharmacology K. L. E. Society’s College of Pharmacy, Bangalore - 560 010 India.
Corresponding Author E-mail: purnimaash06@gmail.com
ABSTRACT: Hygrophila Spinosa (K. Schum) Heine (syn. Asteracantha longifolia Nees, Acanthaceae) was widely used in the Indian systems of medicine for the treatment of various liver ailments. The hepatoprotective activity of the aqueous extract of the stem was studied on carbon tetrachloride induced liver toxicity in rats. The activity was assessed by monitoring the various liver function tests, viz. alanine transaminase, aspartate transaminase (AST), alkaline phosphatase (ALP), total protein and total bilirubin. Furthermore, hepatic tissues were subjected to histopathological studies. The stem extract was also studied for its in vitro antioxidant activity using ferric thiocyanate (FTC) and thiobarbituric acid (TBA) methods. The extract exhibited significant hepatoprotective and antioxidant activities.
KEYWORDS: Hepatoprotective; Antioxidant; Hygrophila spinnosa; Ferric thiocyanate; Superoxide radical
Download this article as:| Copy the following to cite this article: Kshirsagar A. D, Ashok P. Fight Against the BruchHepatoprotective and Antioxidant Effects of Hygrophila Spinosa (K. Schum)Heine Acanthaceae Stem Extract. Biosci Biotechnol Res Asia 2008;5(2) |
| Copy the following to cite this URL: Kshirsagar A. D, Ashok P. Fight Against the BruchHepatoprotective and Antioxidant Effects of Hygrophila Spinosa (K. Schum)Heine Acanthaceae Stem Extract. Biosci Biotechnol Res Asia 2008;5(2). Available from: https://www.biotech-asia.org/?p=7218 |
Introduction
Liver is the most important organ, which plays a pivotal role in regulating various physiological processes in the body. It is involved in several vital functions, such as metabolism, secretion and storage. It has great capacity to detoxicate toxic substances and synthesize useful principles. Therefore, damage to the liver inflicted by hepatotoxic agents is of grave consequences 1, 2. Liver diseases are mainly caused by toxic chemicals, excess consumption of alcohol, infections and autoimmune disorders. Most of the hepatotoxic chemicals damage liver cells mainly by inducing lipid peroxidation and other oxidative damages 3, 4, 5. Inspite of tremendous advances in modern medicine, there are no effective drugs available that stimulate liver function, ate hepatic cells 6. In absence of reliable liver-protective drugs in modern medicine, there are a number of medicinal preparations in ayurveda recommended for the treatment of liver disorders7 and their usage is in vogue since centuries and are quite often claimed to offer significant relief. Hygrophila spinosa (K. Schum) Heine (syn.) Asteracantha longifolia Nees, Acanthaceae are described in ayurvedic literature as Ikshura, Ikshugandha and Kokilasha “having eyes like the Kokila or Indian Cuckoo”. The plant is widely distributed throughout India, Srilanka, Burma, Malaysia and Nepal. The roots, seeds and ashes of the plant are extensively used in traditional system of medicine for various ailments like jaundice, hepatic obstruction, rheumatism, inflammation, pain, urinary infections, edema and gout. It is classified in ayurvedic system as seethaveeryam, mathuravipaka and used for the treatment of premeham (diabetes), athisaram (dysentery), etc. 8, 9. The plant is known to possess antitumor 10, 11, hypoglycemic 12, antibacterial 13, 14, free radical scavenging and lipid peroxidation 15 activities .The seeds are reported for hepatoprotective activity in thioacetamide and paracetamol-induced liver damage in rats16. The literature survey revealed that there are no scientific studies carried out regarding hepatoprotective activity of the stem of Hygrophila spinosa. Hence the present study is focused to evaluate the hepatoprotective and antioxidant potentials of the aqueous extract of Hygrophila spinosa stem (HPSA) against CCl4 and ethanol induced liver injury in rats.
Collection of Plant Material
The HPSA was purchased from Regional Research Institute (Ay.)Govt. Central Pharmacy Annexe,Jayanagar, Bangalore-560011, stored in herbarium with reference no. Ref.8-7/ Drug Supply/ RRI/BNG/2006-07/326.
Preparation of plant extract
The HPSA was defatted with petroleum ether and chloroform. Then extracted with triple distilled water at 50 0C, the yield obtained was 109.2 gm (i.e. 10.92%). The fraction was vacuum dried and stored at – 40 C in deep freezer until used.
Animals
Wistar albino rats (140±20 g) of either sex were housed in large polypropylene cages in a temperature-controlled room (22±2 ◦C) and provided with standardized pelleted feed and clean drinking water ad libitum. The study has got the clearance from the Institutional Animal Ethical Committee (IAEC) of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Acute Toxicity Study
Wistar albino mice were divided into five groups of six animals each. First group served as normal control. The HPSA was administered orally to different groups at the dose level of 250, 500, 1000 and 2000 mg/ (kg p.o.) body weights. All animals were observed for toxic symptoms and mortality for 72 h.
Carbon Tetrachloride Induced Hepatotoxicity
The animals were divided into five groups of six animals each .Group I served as normal control and received distilled water (1 ml/kg, p.o.) daily for 7 days and received olive oil (1 ml/kg, s.c.) on days 2 and 3 17. Group II served as CCl4 control and received distilled water (1 ml/kg, p.o.) daily for 7 days and received CCl4: olive oil (1:1, 2 ml/kg, s.c.) on days 2 and 3. Group III was treated with the reference drug Silymarin (50 mg/kg, p.o.) daily for 7 days and received CCl4: olive oil (1:1, 2 ml/kg, s.c.) on days 2 and 3, 30 min after administration of reference drug. Groups IV–V were treated with HPSA at doses of 250 and 500 mg/(kg p.o.), respectively, for 7 days and received CCl4:olive oil (1:1, 2 ml/kg, s.c.) on days 2 and 3, 30 min after administration of extract.
Biochemical estimations
The rats were sacrificed after individual protocol by cervical decapitation and blood was collected in plain tubes. The serum was obtained by centrifugation. The activities of serum aspartate transaminase (AST) and alanine aminotransferase (ALT) were assayed by the method of King 18. The activities of alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) were estimated by the method of King 19, 20, respectively. The level of serum bilirubin was assayed by the method of Malloy and Evelyn 21. The total protein level was estimated by the method of Reinhold 22.
Histopathological examination
The liver tissues were dissected out and fixed in 10% formalin. The paraffin sections were prepared and stained with haematoxylin and eosin and examined using light microscopy.
Estimation of in Vitro Antioxidant Activity
Ferric thiocynate (FTC) method
The methods of Mitsuda et al. 23and Osawa and Namiki 24 were slightly modified by Kikuzaki and Nakatani 25. A mixture of (25, 50, 75µg/ml) sample in 4ml absolute ethanol, 4.1 ml of 2.52% linoleic acid in absolute ethanol, 8ml of 0.05M phosphate buffer (pH 7.0) and 3.9 ml of water was placed in a test tube with a screw cap and then placed in an oven at 40 ◦C in the dark. To 0.1 ml of this solution 9.7 ml of 75% ethanol and 0.1 ml of 30% ammonium thiocyanate was added. Precisely 3 min after the addition of 0.1 ml of 0.02M ferrous chloride in 3.5% hydrochloric acid to the reaction mixture, the absorbance was measured at 500 nm for every 24 h until the absorbance of the control reached maximum. The control and the standard were subjected to the same procedures as the sample except that for the control, only the solvent was added, and for the standard, 4mg sample was replaced with 4mg of Vitamins C and E.
Superoxide anion radicals scavenging activity (SOD)
Measurement of Superoxide anion radical scavenging activity was based on the method described by Liu et al26 with slight modification. Superoxide radical are generated in phenazine methosulphate – nicotinamide adenine dinucleotide (PMS -NADH) system by oxidation of NADH and assayed by reduction of NBT (nitroblue tetrazolium) In these experiment, the superoxide radicals were generated in 3 ml of Tris-HCl buffer (16mM, pH 8.0) containing 1 ml of NBT (50 um) solution, 1 ml NADH (78um) solution and HPSA (25, 50, 75µg/ml) in water. The reaction started by adding 1ml of PMS solution (10 uM) to mixture. The reaction mixture was incubated at 25 0 C for 5 minute, and the absorbance was measured at 560 nm against blank samples. L- Ascorbic acid was used as a control. Decreased absorbance of the reaction mixture indicated the increasing of Superoxide scavenging activity. The percentage inhibition of Superoxide anion generation was calculated using the following formula.
Percent inhibition = [(A0 − A1) / A0] × 100
Where A0 is the absorbance of control and A1 is the absorbance of sample or standard27.
 |
Graph 1: Invitro antioxidant activity of HPSA by FTC method.
|
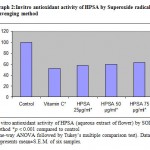 |
Graph 2: Invitro antioxidant activity of HPSA by Superoxide radical scavenging method.
|
Results
The extract revealed the presence of various phytoconstituents, like reducing and non-reducing sugars, flavonoids, sterols and triterpenes. Alkaloids, protein and amino acids were found to be absent.
In acute toxicity, no mortality was observed up to a dose level of 2000 mg/kg body weight. As per the ranking system European Economic Community (EEC) for acute oral toxicity, the LD50 dose of 2000 mg/kg and above is categorized as unclassified (EC Directive 83/467/EEC, 1983).
The activities of various biochemical enzymes in normal, CCl4 control and treated groups were represented in Table 1. The activities of ALT, AST, ALP, LDH and total bilirubin were significantly (p < 0.001) increased with a significant decrease in total protein levels in CCl4 control compared to normal control. The levels of the above enzymes were significantly reversed on treatment with HPSA in a dose-dependent manner. The activity of the extract at the dose of 250 mg/kg was comparable to that of the reference drug silymarin. The histopathological studies of the liver showed fatty changes, swelling and necrosis with loss of hepatocytes in CCl4 control rats in comparison with normal control(Fig: II and II). The HPSA treated groups showed regeneration of hepatocytes, normalization of fatty changes and necrosis of the liver (Fig: IV). The Silymarin treated group showed almost normalization of fatty accumulation and necrosis (Fig: III).
Table 1: Biochemical estimation of serum ALT, AST, LDH, total protein and bilirubin.
| Groups | ALT | AST | ALP | LDH | Total protein | Total bilirubin |
| (IU/l) | (IU/l) | (IU/l) | (IU/l) | (g/dl) | (g/dl) | |
| I | 172.54 ± 10.17 | 88.29 ± 9.77 | 171.92± 2.96 | 177.54± 4.73 | 7.39± 0.07 | 0.65± 0.02 |
| II | 294.32 ± 8.06a* | 235.71 ± 7.59a* | 278.53± 6.51a* | 296.63± 7.98a* | 2.98± 0.09a* | 1.81± 0.03a* |
| III
b* |
225.29 ± 8.22a*b*
|
133.82 ± 10.58a*b*
|
205.56± 7.61a # b*
|
209.74± 8.24a*b*
|
5.85± 0.06a #
|
0.72± 0.05a@b* |
| IV | 260.43 ± 10.65a*b# | 198.10 ± 9.57a*b* | 247.56± 4.69a*b* | 262.74± 5.63a*b* | 4.85± 0.06a*b* | 1.30± 0.04a*b* |
| V | 239.88 ± 11.52a*b* | 161.06 ± 6.78a*b* | 225.47± 4.66a*b* | 236.24± 6.37a*b* | 5.12± 0.06a*b* | 1.06± 0.04a*b* |
Values are mean ± S.E.M. of six animals; symbols represent statistical significance: *p < 0.001, #p < 0.01 and @p < 0.05.
a Comparisons were made between group I vs. groups II–V.
b Comparisons were made between group II vs. groups III–V.
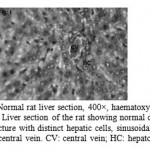 |
Figure 1: Normal rat liver section, 400×, haematoxylin–eosin stain. Liver section of the rat showing normal cellular architecture with distinct hepatic cells, sinusoidal spaces and central vein. CV: central vein; HC: hepatocyte.
|
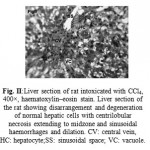 |
Figure 2: Liver section of rat intoxicated with CCl4, 400×, haematoxylin–eosin stain. Liver section of the rat showing disarrangement and degeneration of normal hepatic cells with centrilobular necrosis extending to midzone and sinusoidal haemorrhages and dilation. CV: central vein, HC: hepatocyte;SS: sinusoidal space; VC: vacuole.
|
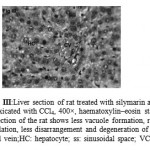 |
Figure 3: Liver section of rat treated with silymarin and intoxicated with CCl4, 400×, haematoxylin–eosin stain. Liver section of the rat shows less vacuole formation, reduced sinusoidal dilation, less disarrangement and degeneration of hepatocytes. CV: central vein;HC: hepatocyte; ss: sinusoidal space; VC: vacuole. |
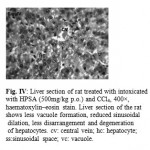 |
Figure 4: Liver section of rat treated with intoxicated with HPSA (500mg/kg p.o.) and CCl4, 400×, haematoxylin–eosin stain. Liver section of the rat shows less vacuole formation, reduced sinusoidal dilation, less disarrangement and degeneration of hepatocytes. cv: central vein; hc: hepatocyte; ss:sinusoidal space; vc: vacuole. |
The antioxidant activity exhibited by HPSA in FTC and Superoxide radical scavenging model (in vitro) is represented in Graph 1 and 2 respectively.
Discussion
CCl4 is biotransformed by the cytochrome P-450 in the liver endoplasmic reticulum to the highly reactive trichloromethyl free radical. This free radical in turn reacts with oxygen to form a trichloromethylperoxy radical, which may attack lipids on the membrane of endoplasmic reticulum more readily than the trichloromethyl free radical. The trichloromethylperoxy radical leads to elicit lipid peroxidation, the disruption of Ca2+ homeostasis, elevation of hepatic enzymes and finally results in cell death 28. The results obtained from the present study indicate that the HPSA exhibited hepatoprotective effect against CCl4-induced liver damage in a dose-dependent manner by normalizing the elevated levels of the hepatic enzymes. Histopathological observations also support the hepatoprotective potential of HPSA. It has been hypothesized that one of the principal causes of CCl4-induced liver injury is lipid peroxidation induced by free radical derivatives of CCl4. Thus, antioxidant activity or the inhibition of the generation of free radicals is important in the protection against CCl4-induced liver injury 29, 30.
FTC method was used to determine the amount of peroxide generated at the initial stage of lipid peroxidation. During the linoleic acid oxidation, peroxides formed and these compounds oxidize Fe2+ to Fe3+. The Fe3+ ions form complex with SCN−, which has a maximum absorbance at 500 nm. In this method, the concentration of peroxide decreases as the antioxidant activity increases. The HPSA exhibited significant antioxidant activity at a concentration of 25µg, 50µg and 75µg compared to control Vitamins C. Lower the absorbance values exhibited higher the antioxidant activities of the samples. The control had the highest absorbance value, followed by vitamin C and HPSA extract at different concentrations. Based on the results HPSA had the shown the concentration dependent increase in the antioxidant activity, the 75µg/ml has shown 68% i.e. highest Invitro antioxidant activity in FTC method (Graph: 1).
Superoxide is biologically important since it can be decomposed to form stronger oxidative species such as singlet oxygen and hydroxyl radicals, is very harmful to the cellular components in a biological system 31. The superoxide anion radical scavenging activity of the extract from HPSA assayed by the PMSNADH system is shown in Graph: 2. The Superoxide scavenging activity of HPSA was increased markedly with the increase of concentrations. These results suggested that HPSA had important superoxide radical scavenging effect. Incidentally the activity of HPSA was found to be better than the standard Vitamins C in both the methods.
The Calotropis gigantea R. Br. reported to contain flavonoids, terpenoids and steol like constituents.32, 33, 34. From the over all results it can be concluded that HPSA exhibited significant antioxidant and hepatoprotective effect against CCl4-induced liver damage which may be due to its anti-lipid peroxidative and free radical scavenging properties. The said potential of HPSA can be attributed to the presence of flavonoids and terpenoids like phytoconstituents in extract. Further a detail investigation is going on in our laboratory to pinpoint the phytoconstituents which is responsible for said potential.
Acknowledgement: Authors are thankful to Mr. B.G. Desai for providing necessary laboratory facilities.
Reference:
- Shahani, S., Indian Drugs, 36, 628–631, (1999).
- Subramoniam, A. and Pushpangadan, P., Indian J of Pharmacol, 31, 166–175, (1999).
- Recknagel, R.O., Life Sciences, 33, 401–408, (1983).
- Wendel, A., Feurensteins, S., Konz, K.H., Biochem Pharmacol, 28, 2051–2053, (1987).
- Dianzani, M.U., Muzia, G., Biocca, M.E., Canuto, R.A., Int J Tissue React, 13, 79–85, (1991).
- Chattopadhyay, R.R., J Ethnopharmacol, 89, 217–219, (2003).
- Chatterjee, T.K., Medicinal Plants with Hepatoprotective Properties, in Herbal Opinions, Books & Allied (P) Ltd., Calcutta, p. 135, (2000).
- Nadkarni, K.M., Indian Materia Medica, Vol. I, Popular Prakashan, Bombay, 667–669, (1978).
- Kirtikar K. R. and Basu N. ,Indian Medicinal Plants,Vol. III , Lolit Mohan Basu , Allahabad, 1864-5.(1935).
- Mazumdar, U.K., Gupta, M., Maiti, S., Mukhejee, D., Indian journal of experimental biology, 35, 473–477, (1997).
- Ahmed, S., Rahman, A., Mathur, M., Athur, M., Sultana, S., Food Chem Toxicol, 39, 19–28, (2001).
- Fernando, M.R., Nalinie Wickramasinghe, S.M.D., Thabrew, M.I., Ariyananda, P.L., Karunanayake, E.H., J Ethnopharmacol, 31, 277–282, (1991).
- Boily, Y., Vanpuyvelde, L., J Ethnopharmacol,16, 1–13, (1986).
- Vlietinck, A.J., Vanhoof, L., Totte, J., Lasure, A., Vanden Berghe, D., Rwangabo, P.C., Mvukiyumwami, J., J Ethnopharmacol, 46, 31–47, (1995).
- Vijayakumar, M., Govindarajan, R., Shriwarkar, A., Kumar, V., Rawat, A., Mehrotra, S., Pushpangadan, P., Natural Product Sciences, 11, 22–26, (2005).
- Singh, A., Handa, S.S., J Ethnopharmacol, 49, 119–126, (1995).
- Rasheeduz Zafar, S., Mujahid, A., J Ethnopharmacol, 63, 227–231, (1998).
- King, J., In: Van, D. (Ed.), Practical Clinical Enzymology. Nostrand Company Limited, London, pp. 191–208,(1965b) .
- King, J., The hydrolases-acid and alkaline phosphatases. In: Van, D. (Ed.), Practical Clinical Enzymology. Nostrand Company Limited, London, pp. 191–208, (1965a).
- King, J., The dehydrogenases or oxidoreductase-lactate dehydrogenase. In: Van, D. (Ed.), Practical Clinical Enzymology. Nostrand Company Limited, London, pp. 83–93, (1965c).
- Malloy, H.T., Evelyn, K.A., J Biol Chem, 119, 481, (1937).
- Reinhold, J.G., In: Reiner, M. (Ed.), Standard Method in Clinical Chemistry, vol. I. Academic Press, New York, p. 88, (1953).
- Mitsuda, H., Yasumoto, K., Iwani, K., Eiyo to Shoduryou ,19, 210(1967).
- Osawa, T., Namiki, M., Agricultural Biological Chemistry, 45, 735–740(1981).
- Kikuzaki, H., Nakatani, N., Journal of Food Sciences 58, 1407–1410(1993).
- Liu F., C.Ooi V. E., and S. T.Chang. , Life Sci. 60, 763-771 (1997).
- Gulcin I., Sat I. G., Beydemir S., Kufrevioglu O .I., Ital J Food Sci,16,17-30,(2004).
- Clawson, G.A., Pathology and Immunopathology Research, 8, 104–112,(1989).
- Castro, J.A., Ferrya, G.C., Castro, C.R., Sasama, H., Fenos, O.M., Gillette, J.R., Biochem Pharmacol, 23, 295–302(1974).
- Maling, H.M., Eichelbaum, F.M., Saul, W., Spies, I.G., Brown, G.A.B., Gillette, J.R., Biochem Pharmacol, 23, 1479–1491(1974).
- Okhawa H., Ohishi N., K. Yagi. Anal Biochem 95, 351-358 (1979).
- Balraj P., Nagrajan S., Indian drug, 19,150(1982).
- Gupta D.R., Bhushan R, Ahmad B., J Nat Prod,46,938(1983)
- Misra TN, Singh RS, Sharma SC, Pandey RP, Indian J. Chem. Sec. B, 39B, 480 (2000).

This work is licensed under a Creative Commons Attribution 4.0 International License.





