How to Cite | Publication History | PlumX Article Matrix
M. Arun Kumar*, S. Sheik Abdullah, K. R. Ramasamy and S. Revathi
Department of Chemistry and Biosciences, Microbiology Divison, Sastra University Srinivasa Ramanujan Centre Kumbakonam - 612 001 India.
Corresponding Author E-mail: microarun2008@yahoo.in
ABSTRACT: Saccharomyces cerevisiae and Zymomonas mobilis immobilized in calcium alginate beads were used in the continuous production of ethanol. They were grown in medium supplemented with ethanol to screen for a culture, which showed greater than 4% tolerance to ethanol inhibition. Beads were produced from culture slurry containing 2% alginate (w/v), which has added as drops to 2% calcium chloride solution. The immobilized- cell column was operated continuously at steady state for 10 cycles. The yield of ethanol in the immobilized cell system was higher than in free-cell system. To determine their optimum fermentation parameters ethanol production using glucose as a substrate was monitored in continuous systems at varying gel concentrations. Ethanol production rates, as well as residual sugar concentrations were monitored. Rapid fermentation of cane molasses into ethanol has been studied in continuous (free cell and cell- immobilized system). However the removal and discard of used cells remained a problem in the alcoholic fermentations. This obstacle was removed by carefully immobilizing the cells using calcium alginate. Under optimized conditions fermentation was carried out using immobilized cells and good results were obtained. Same immobilized cells after first fermentation were used for next cycle and results were acceptable. The ethanol yield was found to be higher in immobilized Saccharomyces cerevisiae than immobilized Zymomonas mobilis.
KEYWORDS:
Continuous fermentation; sodium alginate; Saccharomyces cerevisiae; Zymomonas mobilis
Download this article as:| Copy the following to cite this article: Kumar M. A, Sheik Abdullah, S, Ramasamy K. R, Revathi S.Comparative Optimization of Ethanol Production Using Immobilized Saccharomyces Cerevisiae and Zymomonas Mobilis. Biosci Biotechnol Res Asia 2009;6(1) |
| Copy the following to cite this URL: Kumar M. A, Sheik Abdullah, S, Ramasamy K. R, Revathi S.Comparative Optimization of Ethanol Production Using Immobilized Saccharomyces Cerevisiae and Zymomonas Mobilis. Biosci Biotechnol Res Asia 2009;6(1) Available from: https://www.biotech-asia.org/?p=7996 |
Introduction
In India, ethanol is produced by the fermentation of diluted cane molasses using Saccharomyces Cerevisiae. There are 285 distilleries in the country with an installed capacity over 2.7 billion liters of ethanol annually. Because of energy crisis, interest has been shown in producing ethanol from renewable sources. Ethanol is an important industrial solvent and a chemical feed stock for the synthesis of pharmaceuticals, detergent adhesives, plastics, plasticizers and host for other chemicals. It is also an energy feed stock and as a fuel in internal combustion engines1. In India the production of ethanol is exclusively from the fermentation of cane molasses. Molasses is one of the most important raw materials used for ethanol production in majority of distilleries in India. The composition and properties of molasses show considerable degree of variation depending upon the source, season and conditions of storage2.
Due to dwindling of fossil fuel, microbial production of bio-fuel from organic byproducts has acquired significance in recent years. Ethanol has been trusted as an alternate fuel for the future. Even though several microorganisms, including Clostridium sp., have been considered as ethanologenic microbes, the yeast Saccharomyces cerevisiae and facultative bacterium Zymomonas mobilis are better candidates for industrial alcohol production. Saccharomyces cerevisiae is a species of budding yeast. It is perhaps the most useful yeast in baking and brewing. Saccharomyces cerevisiae cells are round to ovoid, 5–10 micrometers in diameter These organisms have long been utilized to ferment the sugars of rice, wheat, barley, and corn to produce alcoholic beverages and in the baking industry to expand, or raise, dough. Yeast is often taken as a vitamin supplement because it is 50 percent protein and is a rich source of B vitamins, niacin, and folic acid. Ethanol in India and other developing countries is mainly produced by fermentation of dilute molasses at ambient temperature of 25ºC-35ºC employing Saccharomyces cerevisiae.
Zymomonas mobilis is a bacterium belonging to the genus Zymomonas. It is notable for its bioethanol-producing capabilities, which surpass yeast in some aspects. It was originally isolated from alcoholic beverages like the African palm wine, the Mexican pulque, and also as a contaminant of cider and beer in European countries. Zymomonas mobilis degrades sugars to pyruvate using the Entner-Doudoroff pathway. The pyruvate is then fermentated to produce ethanol and carbon dioxide as the only products. The advantages of Zymomonas mobilis over Saccharomyces cerevisiae with respect to producing bioethanol:
Higher sugar uptake and ethanol yield,
Lower biomass production,
Higher ethanol tolerance,
Does not require controlled addition of oxygen during the fermentation,
Amenability to genetic manipulations.
However, it has a severe limitation compared to yeast its utilizable substrate range is restricted to glucose, fructose, and sucrose. Using biotechnological methods, scientists are currently trying to overcome this. A variant of Zymomonas mobilis that is able to use certain pentoses as a carbon source has been developed. An interesting characteristic of Zymomonas mobilis is that its plasma membrane contains hopanoids, pentacyclic compounds similar to eukaryotic sterols. This allows it to have an extraordinary tolerance to ethanol in its environment, around 13%.
Immobilization is the technique used for the physical or chemical fixation of cells, organelles, enzymes, or other proteins onto a solid support, into a solid matrix or retained by a membrane, in order to increase their stability and make possible their repeated or continued use. Immobilized cells exhibit many advantages over free cells, such as relative ease of product separation, reuse of biocatalysts, high volumetric productivity, improved process control and reduced susceptibility of cells to contamination. Among the different cell immobilization techniques, entrapment in calcium alginate gel has been one of the most used matrices for whole cell entrapment due to its simplicity and non-toxic character3. This simple and mild immobilization technique involves the drop wise addition of cell suspended in sodium alginate on to a solution of calcium chloride where on the cells are immobilized in precipitated calcium alginate gel in the form of beads. Continuous fermentation offers important advantages, such as higher conversion rates, faster fermentation rates, improved product consistency, reduced product losses and environmental advantages.
Materials and methods
Microorganisms and culture condition
Isolation of Saccharomyces cerevisiae
The strain of Saccharomyces cerevisiae used in this work was isolated from spoiled grapes and maintained by sub culturing them every 15 days on YEPD medium (g/l) Yeast extract, 3.0; Peptone, 10.0; Dextrose, 20.0; Agar, 15.0.
Isolation of Zymomonas mobilis
The strain of Zymomonas mobilis used in this work was isolated from palm wine and it was maintained by transfer to the following medium (g/l): sucrose, 50.0; yeast extract, 5.0; KH2PO4, 1.0; (NH4)2SO4, 1.0; MgSO4, 0.50.
Substrate
Sample collection
Molasses used in this study was procured from Thiruarroran sugars limited; Andakudi, Tamilnadu, India and it were estimated for total sugars and total fermentable sugars.
Pretreatment of molasses
Even though molasses contain macro elements like Iron, Zinc, Calcium, Potassium, Magnesium and microelements like Cobalt, Boron, and Cadmium for better production of ethanol, pretreatment and supplementation is required. For that the production medium containing KH2PO4, 0.1%; (NH4)2SO4, 0.5%; MgSO4.7H2O, 0.05%; Yeast extract, 0.1% was supplemented with nitrogen and phosphorus. The pH of the medium was adjusted to 5.0.
Estimation of total sugar
Sugars undergo dehydration in the presence of sulphuric acid to furfural that condenses with phenol to form yellowish orange coloured compound with absorption maximum at 490nm4.
Estimation of reducing sugar
The dinitrosalicyclic acid (DNS) reagent is composed of 3, 5-dinitrosalicyclic acid, Rochelle salt, phenol, sodium sulphite and sodium hydroxide. Rochelle salt is introduced to prevent the reagent from dissolving oxygen; phenol, to increase the amount of colour produced; and sodium sulphite to stabilize the colour obtained in the presence of phenol. Sodium hydroxide is required for the reducing action of glucose on dinitrosalicyclic acid5.
Immobilization
Entrapment materials
In this study, sodium alginate and calcium chloride was used as entrapment materials for better yield of ethanol in continuous fermentation process.
Standardization of alginate concentration
In this experiment an attempt had been done to stabilize alginate concentration by keeping the calcium chloride concentration as the constant one. It was noted that rupture of beads occurs in certain series of fermentation cycle due to the production of ethanol. After repeated analysis it was observed that 2% of sodium alginate was optimal. The beads were thick and also there was no rupture of beads occurs even after 10th cycle of production.
Standardization of calcium chloride concentration
A series of experiment were performed by keeping a concentration of sodium alginate as constant (2%) with an increasing concentration of calcium chloride like 1.5%, 2.0%, and 2.5%. fermentation process was carried out for the different concentrations of calcium chloride and the maximum ethanol producing concentration with out rupture of beads was used for continuous production of ethanol.
Calcium alginate entrapment of yeast cells
The calcium alginate gel-entrapping method was preferred because of its high enzymatic activity, simple manner of preparation and stability. The preparation of calcium alginate beads does not require the use of specialized equipment. They were readily obtained by adding sodium alginate solution to calcium chloride solution6.
Fermentation
Fermentation process was initiated with the inoculation of immobilized yeast cells to the pretreated molasses in sterile Erlenmeyer flasks under aseptic condition. The flasks were incubated under shaking condition at room temperature for 10-15 days. After 15 days ethanol produced was distilled (at 78.5ºC) and estimated. The process was repeated for 10 fermentation cycles.
Estimation of ethanol
The percentage of ethanol produced in the fermentation medium was estimated using colorimetric method7. Dichromate is yellowish in color and the reduced chromic product is intensely green. The green colour developed was read at 600nm.
Results
Molasses the by-product of sugar industry is mainly used as substrate for ethanol production. Estimation of total sugar and reducing sugar in molasses was found to be important for efficient production of ethanol. In this work the total sugar content of molasses was estimated by phenol suphuric method4 and found to be 45% of total sugar. Total reducing sugar of the molasses was estimated by DNS (Dinitrosalicyclic acid) method and was found to be 10.8%. The isolated strain of Saccharomyces cerevisiae and Zymomonas mobilis were grown in the medium supplemented with ethanol, the screened cultures shows 4% and 3% tolerance to ethanol inhibition respectively.
The optimization of sodium alginate was done as shown in table-1. From the observed results 2% and 2.5% of sodium alginate with 2% of calcium chloride gave stable beads compared to other concentrations. From that 2% of sodium alginate was used to standardize calcium chloride. Estimation of ethanol from optimized sodium alginate was done as shown in table-2. By preparing different concentration of sodium alginate (2%, 3%, and 4%) with constant calcium chloride concentration (2%) and high yield of ethanol (7.1%) was obtained from 2% of sodium alginate and calcium chloride.
The calcium chloride concentration was standardized as shown in table-3. From the observed results it was clear that 2% of sodium alginate and calcium chloride produced high yield of ethanol (7.1%) and the beads were found to be highly stable. After carrying out immobilization of Saccharomyces cerevisiae, the immobilized beads were used to carry out the fermentation. After 15 days of fermentation the samples were filtered and the beads were removed. Table-4 explained the production of ethanol from immobilized yeast cells with optimized concentration of sodium alginate and calcium chloride.
From all the observed results, the prepared beads of Saccharomyces cerevisiae were found to be highly stable through out 10 fermentation cycles and produced nearly 7.1% of ethanol, and beads of Zymomonas mobilis produce 6% of ethanol Figure-1.
Table 1: Optimization of Sodium Alginate Concentration.
|
S.NO
|
Concentration of calcium chloride (%) | Concentration of sodium alginate (%) | Stability of beads |
| 1 | 2.00 | 1.00 | Not stable |
| 2 | 2.00 | 1.50 | Not stable |
| 3 | 2.00 | 2.00 | Fine |
| 4 | 2.00 | 2.50 | Fine |
| 5 | 2.00 | 3.00 | Little hard |
| 6 | 2.00 | 3.50 | Little hard |
| 7 | 2.00 | 4.00 | Hard |
| 8 | 2.00 | 4.50 | Hard |
| 9 | 2.00 | 5.00 | Too hard |
Table 2: Ethanol Production from Optimized Sodium Alginate Concentration.
| S.NO | Concentration of sodium alginate (%) | Stability of beads | Percentage of ethanol
(%) |
| 1 | 2.00 | Fine | 7.1 |
| 2 | 3.00 | Little hard | 6.4 |
| 3 | 4.00 | Hard | 5.6 |
Table 3: Optimization of Calcium Chloride Concentration.
|
S.NO
|
Concentration of sodium alginate (%) | Concentration of calcium chloride (%) | Stability of beads |
| 1 | 2.00 | 1.00 | Beads broken |
| 2 | 2.00 | 1.50 | Beads broken |
| 3 | 2.00 | 2.00 | Fine |
| 4 | 2.00 | 2.50 | Fine |
| 5 | 2.00 | 3.00 | Little hard |
| 6 | 2.00 | 3.50 | Little hard |
| 7 | 2.00 | 4.00 | Hard |
| 8 | 2.00 | 4.50 | Hard |
| 9 | 2.00 | 5.00 | Too hard |
Table 4: Ethanol Production from Optimized Sodium Alginate and Calcium Chloride.
| S.NO | Concentration of sodium alginate (%) | Concentration of calcium chloride (%) | Stability of beads | Percentage of ethanol
(%) |
| 1 | 2.00 | 1.50 | Fine | 6.8 |
| 2 | 2.00 | 2.00 | Fine | 7.1 |
| 3 | 2.00 | 2.50 | Little hard | 6.7 |
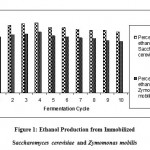 |
Figure 1: Ethanol Production from Immobilized Saccharomyces cerevisiae and Zymomonas mobilis.
|
Discussion
The ethanol fermentation process is dependent on environmental parameter used. The need to understand the physiological regulation of yeast for ethanol production is important when using standard fermentation process and has been studied in detail for many years. Yeasts that are trapped in an immobilizing matrix are subjected to a much different environment than cells in a free suspension, and little information concerning yeast metabolism by cells bound in as matrix has been obtained. Therefore, one of the objectives of this work was to define some of the factors necessary for maximum ethanol production rates.
The reduction of metabolic activity even when sufficient nutrients are available can be caused by metabolic end- products and in the case of ethanol fermentation inhibition is caused by the ethanol itself8. The yeast culture used in the immobilized whole cell process was selected on the basis of growth rate as an indicator of ethanol tolerance. Therefore, growth rates in high concentrations of ethanol tolerance in a simple screening procedure.
The preparation of alginate- yeast beads by the calcium alginate entrapment technique is a simple and gentle method for whole cell immobilization. Alginate beads possess good mechanical strength and can withstand packing pressure without extensive damage. Alginate is resistant to acidic and basic environments and is stable even at thermophilic fermentation temperatures9. The scale up of the immobilized yeast cell process to industrial scale would involve several economic evaluations. The cost of substrate is the greatest single expense in ethanol fermentation and is estimated to represent more amount of ethanol production nearly for 10 fermentation cycles. In this work Saccharomyces cerevisiae and Zymomonas mobilis were isolated from spoiled grapes and palm wine respectively and used for continuous ethanol production by immobilization technique Saccharomyces cerevisiae were isolated from toddy and obtained optimum ethanol concentration of 48g/l at the optimum temperature at 30ºC and pH 4.2510.
Molasses act as an important substrate for ethanol production. Saccharomyces cerevisiae immobilized in agar gel and used in a tubular reactor for conversion of cane molasses to ethanol at 30ºC, pH, 4.5. Reactor was used in a continuous operation to test the operational stability and ethanol productivity. Maximum ethanol concentration (94.9g/l) was obtained with a feed containing 255g/l reducing sugar11. The continuous production of ethanol from beet molasses by calcium alginate immobilized Saccharomyces cerevisiae in a packed- bed bioreactor was monitored and temperature was maintained at 30ºC and the dilution rate was 0.22 h-1. Maximum ethanol (4.62%), theoretical yield (82.9%) and volumetric productivity (10.16g/l) were obtained from the beet molasses medium containing 10.90% total sugar with 2.0-2.4mm diameter beads from 2% (w/v) sodium alginate solution6.
The rapid fermentation of cane molasses into ethanol has been studied in batch, continuous by a strain of Saccharomyces cerevisiae at temperature 30ºC and pH 5.0. The cells were immobilized by natural mode on a carrier of natural origin and retention of 0.132g cells/g carrier was achieved12. For efficient ethanol yield molasses were pretreated using KH2PO4, (NH4)2SO4, MgSO4 and yeast extract. The effect of pretreatment of molasses with H2SO4 and K4Fe(CN)6 on ethanol production by different yeast strains is a effective method to reduce the presence of various inhibitory substances and to select a suitable yeast strain for fermentation of pretreated molasses.
Saccharomyces cerevisiae was widely used for ethanol production because of its high fermenting ability under optimized conditions. They can able to tolerate high concentration sugar and can able to tolerate 4% of ethanol. The strain of Saccharomyces cerevisiae used in this study was a good alcohol producer isolated from spoiled grapes. Maximum amount of ethanol (7.5%) was obtained using immobilized Saccharomyces cerevisiae.
The recombinant Zymomonas mobilis able to efficiently ferment glucose and xylose at a relatively high concentration (12–15%), that is a typical hydrolysate produced from cellulosic feedstocks. Even though several microorganisms, including Clostridium sp., have been considered as ethanologenic microbes, the yeast Saccharomyces cerevisiae and facultative bacterium Zymomonas mobilis are better candidates for industrial alcohol production. Zymomonas mobilis possesses advantages over S. cerevisiae with respect to ethanol productivity and tolerance, thus encouraging researchers for exploiting Zymomonas mobilis ability to utilize sucrose, glucose, and fructose by Entner–Deudoroff pathway13.
Among various types of immobilization carriers calcium alginate was widely used because of its stability and less toxicity. In this study calcium alginate beads were prepared using 2% of sodium alginate and 2% of calcium chloride and found to be highly stable for 10 fermentation cycles with high yield of ethanol (7.1%). Saccharomyces cerevisiae immobilized on calcium alginate to carry out the fermentation studies using glucose as a substrate was monitor in continuous system. Immobilization of yeast cells (10-40% w/v) in 2% calcium alginate was accomplished and these were tested for ethanol production at varying temperature and pH of the feed. Maximum ethanol productivity (7.1%) was achieved at 30ºC, pH 4.5 at a dilution rate 0.20/h. The concentration of sodium alginate and calcium chloride play vital role in determination of bead stability leads to efficient ethanol productivity.
References
- Rekha Dabas, Verma VK & Kamla Chaudhary. 1997. Ethanol production from wheat starch. Indian Journal of Microbiology 37: 49-50.
- Sharma S &Tauro P. 1986. Control of ethanol production by Saccharomyces cerevisiae. Journal of Chemical Engineering 234-238.
- Singh D, Nigam P, Merchant R and Mchole AP. 1998. Ethanol production at elevated temperatures and alcohol concentration: Part 2- use of Kluyveromyces maxianus IMB3. World Journal of Microbial biotechnology. 14: 823-34.
- Dubois M, Rebers P A & Smith. 1956. Estimation of total sugar using phenol sulphuric method. Journal of Analytical Chemistry 114-115.
- Miller G L. 1959. Use of dinitrosalicyclic acid reagent for determination of reducing sugar. Analytical Chemistry 31 : 426-428.
- Yekta Goksungur & Nese Zotlu. 2001. Production of ethanol from beet molasses by calcium alginate immobilized yeast cells in a packed bed bioreactor. Turkian Journal of Biology 25: 265-275.
- Caputi A, ueda M and BrownT. 1968. Spectrophotometric determination of ethanol in wine. American journal of Enology viticulture. 19: 160-165.
- Dahiya DS and Rose AH. 1986. Factors affecting ethanol sensitivity and production by yeasts. Yeast Biotechnology 37-48.
- Lida T. (1993). Fuel ethanol production by immobilized yeast and test immobilization. Bioprocess Technology: in: Industrial Application of Immobilized Biocatalysts. 73-75.
- Pramanik K. (2003). Parametric studies on batch alcohol fermentation using Saccharomyces cerevisiae yeast extracted from toddy. Journal of Chemical Engineering 34: 487-492.
- Jones A.M, Thomas KC and Inglew WM. 1994. Ethanolic fermentation of molasses and sugarcane juice using very high gravity technology. Journal of Agricultural and chemical technology. 42.: 1242-1246.
- Tyagi R D & Ghose T K. (1982). Analysis of continuous rapid ethanol fermentation in immobilized cell reactor. Biotechnology and Bioengineering 24: 781-795.
- Yamada T, Fatigati M A & Zhang M. 1998. Performance of immobilized Zymomonas mobilis 31821 (pZB5) on actual hydrolysates produced by Arkenol technology. Applied Biochemistry Biotechnology 98: 899–907.

This work is licensed under a Creative Commons Attribution 4.0 International License.





