How to Cite | Publication History | PlumX Article Matrix
Studies on Degradation of Diesel and Other Petroleum Fractions by Acinetobacter Junii, CTA3
Sonali Sahoo, Dipa Biswas*, Sriparna Datta and Rakhi Banik Choudhury
Department of Chemical Technology, University of Calcutta, 92, A.P.C Road, Kolkata - 700 009 India.
Corresponding Author E-mail: dipa_bis@yahoo.com
ABSTRACT: Strain CTA3 was isolated from oil contaminated soil and tested with various petroleum fractions /intermediates viz. gasoline, kerosene, diesel, vacuum gas oil (VGO), lubricating oil base stock (LOBS) as well as some pure hydrocarbons. Based on DNA sequence CTA3 was identified as Acinetobacter junii. The isolated strain CTA3 has been found to be very effective for biodegradation of petroleum fractions at 37o in liquid Bushnell-Haas (BH) media at pH 7. The study reveals that the strain has good potential for degrading higher n-paraffins, above C10.
KEYWORDS: Acinetobacter junii; biotreatment; Bushnell-Haas media; diesel oil
Download this article as:| Copy the following to cite this article: Sahoo S, Biswas D, Datta S, Choudhury R. B. Studies on Degradation of Diesel and Other Petroleum Fractions by Acinetobacter Junii, CTA3. Biosci Biotechnol Res Asia 2008;6(1) |
| Copy the following to cite this URL: Sahoo S, Biswas D, Datta S, Choudhury R. B. Studies on Degradation of Diesel and Other Petroleum Fractions by Acinetobacter Junii, CTA3. Biosci Biotechnol Res Asia 2008;6(1). Available from: https://www.biotech-asia.org/?p=8079 |
Introduction
Soil contamination with diesel and other petroleum fractions is being one of the major environmental problems with increasing usage of petroleum hydrocarbon products in the recent days. Thus to reduce the environmental pollution “biotreatment” is now an important field of work. Application of microbiology for such purpose started in 1946 by Zobell and his co-workers1. A diesel degrading bacteria(strain IU5) isolated from oil contaminated soil in Korea2 was found to degrade many other petroleum hydrocarbons including crude oil, gasoline, benzene, toluene, xylene, naphthalene, phenanthrene and pyrene. The different microbial strain, Mycobacterium sp. strain 1B3, Mycobacterium austrofricanum GTI 234, Pseudomonas putida 34, Pseudomonas fluorescens 62, Pseudomonas aeruginosa 57, Sphingomonas sp. strain 1075 were found to be capable of degrading PAH like naphthalene, anthracene, phenanthrene, pyrene6,7 etc. which are very toxic to the environment. Acinetobacter sp. strain DSM 17874 was found to degrade C10 to C40 n-alkanes8. Strains of genus Acinetobacter capable of utilizing C10 to C44 alkanes have been described by the workers9,10,11,12,13. This sp. are widespread in nature and can degrade a wide range of organic compounds such as phenol14,15 , toluene16, o-nitro aniline, o-nitro toluene etc.17 as well as inorganic compounds such as phosphates and metals18,19,20. It was observed by the investigators that, Acinetobacter sp. can degrade 37% oily sludge, the complex mixture of petroleum hydrocarbons (such as alkanes, aromatics, resins and asphaltenes), sediments, heavy metals and water21. Species of Acinetobacter have been gaining attention in both environmental and biotechnological applications22.
We have isolated a strain of Acinetobacter sp. from oil contaminated soil samples and its effect on degrading various petroleum fractions/intermediates was studied.
Materials and Methods
Soil samples, Petroleum fractions / intermediates & other Chemicals
The work started with collection of soil samples from different oil contaminated places in and around Kolkata. Five different soil samples were collected. Petroleum products viz. gasoline, kerosene and diesel were procured from the retail market and intermediates viz. atmospheric gas oil, vacuum gas oil (VGO), diesel hydrodesulfurization (DHDS) feed, lubricating oil base stock (LOBS) were collected from Indian Oil Corporation Ltd., Haldia refinery, W.B., India. Pure hydrocarbons were procured from Emerck. Other chemicals and solvents were of LR grade and purchased from local suppliers.
Media
Media used for the isolation and cultivation of cultures were Bushnell-Haas (BH) and Nutrient agar from Hi Media Laboratories Pvt. Ltd., Mumbai, India. The composition of BH media is MgSO4, 0.2 g; CaCl2, 0.02 g; KH2PO4, 1.0 g; K2HPO4, 1.0g; NH4NO3, 1.0 g; FeCl3, 0.05 g ( pH 7.0 ± 0.2 at 25o) per liter. Nutrient agar media contains peptone 1% w/v, NaCl 0.5% w/v, beef extract 0.5% w/v, agar 2% w/v. (pH 7.2 – 7.4)
Isolation of Microorganism
In a properly sterilized conical flask, containing 50 ml BH media, 10 gm of soil sample was added aseptically and shaken for 5 minutes, it was then allowed to settle for 45 minutes. The clear liquid was then decanted aseptically into another empty sterile conical flask. 2% diesel was added as the sole carbon source for microorganisms. The flask was incubated for four days at 37o in an incubator cum shaker. After four days huge growth was observed. Then isolation of organism was done on nutrient agar plates by streak plate method. Single colonies were marked on the plates and these were separately isolated in the agar slants. The whole process was repeated for all the samples. A large number of organisms were thus isolated and these were tested for their best utilization of diesel using liquid BH media. Organism CTA3 showed maximum growth and ultimately it was selected for further studies. The organism was stored in nutrient agar media at 10o in a refrigerator and subculture on nutrient agar slants were used as inoculums.
Identification of Microorganism
At first gram staining was done with this microorganism. Organism, CTA3 was gram –ve coccus when observed under microscope. Next, the organism was identified based on DNA sequences. For identification DNA from pure cultures was extracted23. The DNA extract was subjected to polymerase chain reaction (PCR) in a thermal cycler (Perkin Elmer 2400, USA) using bacterial forward and backward primer under the following conditions: 5 min at 94o, 40 cycles of 30 sec at 94o, 30 sec at 53o, 45 sec at 72o and one final step of 7 min at 72o24. The amplified DNA fragments were purified using agarose (SRL, India) and a QIA quick spin column kit (Qiagen, Stanford, CA, USA). Purified DNA was sequenced by First BASE Laboratories Sdn Bhd, Malaysia through Agile Lifescience Technologies India Pvt. Ltd, Thane, India. Database searches were conducted with the Blast algorithm provided by the National Center for Biotechnology Information.
Optimization of process parameters
Optimization of the pH with the BH media was done with diesel with the microorganism CTA3 at different pH. After optimizing pH, optimum day of incubation was found by treating 10% diesel with CTA3 and measuring the optical density (O.D.) of liquid broth after 24 hours interval upto 5th day.
Treatment of Diesel
(a) Variation of diesel percentage
Fresh overnight culture of CTA3, was taken to inoculate the sterile BH media containing different percentage of diesel ranging from 5- 25% (v/v) at pH 7. The flasks were kept under shaking condition in a shaker incubator at 37o. The experiment was also repeated with higher percentage (upto 90%) of diesel.
(b) Successive Treatment
It was found that after the treatment of higher percentages of diesel some amount of diesel was left over. Hence we were interested to treat that left over diesel with a fresh lot of culture again. The diesel (10 %) was treated three times successively in the same procedure mentioned above. The treated oil of the first step was treated again with fresh culture and so on. Every time similar huge growth was observed in case of treated oil. Finally after third treatment oil was separated and tested for the changes in corresponding physiochemical properties. A comparative study of the properties was then performed using the untreated diesel and treated diesel after third treatment.
Treatment of other Petroleum fractions/intermediates
After successful treatment with diesel, other petroleum fractions and intermediates viz. gasoline, kerosene, atmospheric gas oil, VGO, DHDS and LOBS were treated with the same microorganism using 10% (v/v) of each oil. Also, for VGO, DHDS and LOBS treatment was done upto 90% of oil.
Treatment of Pure Hydrocarbons
Further to establish the effect of the microorganism on the particular hydrocarbons present in the oil the bacteria was used to treat several pure hydrocarbons like hexane, heptane, nonane, dodecane, hexadecane, isooctane, decalin, benzene, toluene, o-xylene, tetralin, naphthalene, anthracene and phenanthrene of the alkane, cycloalkane and aromatic group.
Test Methods
(a) Physicochemical properties
Standard (ASTM/IP) methods were used for testing the physiochemical properties of the untreated and the treated diesel, e. g., Aniline Point: ASTM D 611 / IP 2; Pour Point: ASTM D 97 / IP 15; Kinematic Viscosity: ASTM D 445 / IP 71
Following correlations were also used for further assessment e.g.,
oAPI = (141.5/sp. gr.(15.5 o /15.5o)) – 131.5
Diesel Index (D.I) = Aniline Point(oF) * oAPI/100
Cetane No. = (0.72*Diesel Index) + 10
Cetane Index = (0.566*Diesel Index) + 21.3
(b) Compositional Analysis
Fluorescent Indicator Adsorption (FIA) chromatography (ASTM D 1319 / IP156) and High Performance Liquid Chromatography (HPLC) analysis were done with the oil samples before and after treatment to observe the changes in hydrocarbon type. HPLC (PerkinElmer S 200) equipped with 4.6×250 mm Peerless-amino column was used to determine total aromatics (%wt) using RI detector. The mobile phase used was n-heptane, at flow rate 1ml/min.
Results
Identification of organism, CTA3
The strain, CTA3 was identified as Acinetobacter junii (GenBank accession no. AM410704) according to its DNA sequences.
Optimization of process parameters
The optimum pH of the liquid BH media for incubation of the organism, CTA3, was found to be 7 at 37o. Any change of pH from that point resulted lower growth of organism. At pH 7 optimum growth of organism CTA3 was observed on the third day of incubation (Fig. 1); after that O.D of liquid broth did not increase significantly.
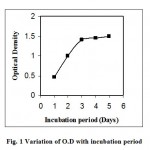 |
Figure 1: Variation of O.D with incubation period.
|
Treatment of various percentage of diesel
During the treatment of different percentage of diesel, after three days incubation, heavy growth was observed in all the flasks containing 5%, 10%, 15%, 20%, 25% of diesel. The organism also showed significant growth with higher percentage of diesel even with 90% diesel content.
Successive Treatment of diesel
After treating the diesel in three successions, the properties of the treated oil were measured and the hydrocarbon composition was also studied. From the comparative study of the properties of diesel (Table 1), it is observed that aniline point, diesel index, cetane no., cetane index, pour pt. values were decreased and sp. gravity, viscosity values were increased for treated oil. The wt% of total aromatics (Table 2) is increased for treated oil when analyzed by HPLC. Analysis of hydrocarbon type by FIA (data not shown) also results an increase in total aromatics in the treated diesel as the HPLC analysis.
Table 1: Comparative Study of Properties of Treated (After 3rd Treatment) & Untreated Diesel.
| Properties | Diesel | |
| Untreated | Treated | |
| Sp. gravity at 15.5 o/15.5o
Kinematic Viscosity at 38o,cSt Aniline Pt. (o) Diesel Index Cetane No. Cetane Index Pour Pt. (o) |
0.8470
3.025 74 58.75 52.30 54.55 – 5.5 |
0.8517
3.659 68.5 53.79 48.73 51.75 – 8 |
Table 2: Analysis of Total Aromatics in Untreated and Treated Diesel by Hplc.
| Diesel | Untreated
(wt%) |
Treated
(wt%) |
| Monoaromatic hydrocarbon (MAH) | 28.9763 | 32.8781 |
| Diaromatic hydrocarbon (DAH) | 3.7507 | 4.0032 |
| Polyaromatic hydrocarbon (PAH) | 0.1019 | 0.1283 |
| Total Aromatics | 32.8289 | 37.0096 |
Treatment of other Petroleum fractions/intermediates
During treatment with other petroleum fractions/intermediates, weak growth was observed in the flask containing gasoline, medium growth was observed in kerosene containing flask whereas, atm. gas oil, VGO, DHDS and LOBS resulted in huge growth (Table 3).
Table 3: Growth Potentials of Cta3 on Some Petroleum Fractions/Intermediates.
| Petroleum fractions/ intermediates | Growth potentials |
| Gasoline
Kerosene Atmosphearic Gas Oil VGO DHDS LOBS |
+
++ +++ +++ +++ +++ |
Characteristics were scored as: +, weak growth; ++, medium growth; +++, heavy growth.
Treatment of Pure Hydrocarbons
The growth characteristics of the organism with pure hydrocarbons of different types are presented in Table 4. The organism Acinetobacter junii CTA3 degraded only dodecane and hexadecane. Other hydrocarbons viz. heptane, nonane, isooctane, decalin, benzene, toluene, o-xylene, tetralin, naphthalene, anthracene and phenanthrene were not degraded by the organism.
Table 4: Growth Potentials of Cta3 on Some Pure Hydrocarbons.
| Hydrocarbons | Hydrocarbon Types | Growth Potentials |
| Hexane
Heptane Nonane Dodecane Hexadecane Isooctane |
Alkane |
–
– – ++ +++ – |
| Decalin | Cycloalkane | – |
| Benzene
Toluene o-Xylene Tetralin Naphthalene Anthracene Phenanthrene |
Aromatic |
–
– – – – – – |
Characteristics were scored as: -, no growth; +, weak growth; ++, medium growth; +++, heavy growth.
Discussion
In this study, microorganism CTA3 was isolated from oil contaminated soil and identified as Acinetobacter junii based on DNA sequence analysis. The experiment with mineral liquid BH media, in absence of any carbon source, confirmed the ability of the organism, CTA3, to grow in petroleum oils like diesel, VGO, LOBS etc. as the sole substrate. The organism showed significant growth even with higher percentage of oil (90 vol%) i.e. growth was independent of the amount of oil/carbon source; i.e. the higher percentage of oil does not inhibit the growth.
From the study of physiochemical properties of treated and untreated diesel, it can be said that the alkanes are depleted in the treated diesel on treatment with the particular microorganism as aniline point, diesel index, cetane no., cetane index, pour pt. values decreased whereas sp. gravity, viscosity values were increased which was in conformity with the result using pure hydrocarbons. This observation also corroborated from the analysis of FIA and HPLC.
Again, from the study of various petroleum fractions and intermediates it can be found that the microorganism, CTA3, is more suitable for treatment of higher boiling petroleum fractions. It was further supported from the experiments using pure hydrocarbons which revealed that only dodecane and hexadecane containing flasks showed growth of organism, although better growth was observed in hexadecane as compared to dodecane. Other hydrocarbon containing flasks showed no growth of organism. This observation established that the selected microorganism is effective for treatment of hydrocarbons of C10 onwards especially the alkanes and was much more effective for higher ones.
The study therefore reveals that the isolated microorganism, CTA3, can successfully utilized for the treatment of less volatile, higher boiling petroleum fractions rich in alkanes or soils contaminated with those fractions in degrading particularly the alkane group of hydrocarbons of C10 onwards. Biotreatment with this organism can thus be an alternative process for improving the pour point of the diesel fraction or for de-waxing the lubricating oil base stock.
Acknowledgement
The authors are thankful to Dr. Maitrayee Dasgupta, Reader, Dept. of Biochemistry, University of Calcutta for her cordial help regarding the DNA sequencing. We highly appreciated the help of Dr. R. K. Mandal, Aglow Quality Control Laboratory Pvt. Ltd., kol- 26 for his assistance. The authors are also grateful to Technical Education Quality Improvement Program (TEQIP) for providing financial support.
References
- Zobell C. E., Bacteriol. Rev., 10, 149 (1946).
- Hong J. H., Kim J., Choi O. K., Cho K. S. and Ryu H. W., World J. Microbiol. Biotech., 21, 381-384 (2005).
- Dandie C. E., Thomas S. M., Bentham R. H. and McClure N. C., J. Appl. Microbiol., 97, 246-255 (2004).
- Bogan B. W., Lahner L. M., Sullivan W. R. and Paterek J. R., J. Appl. Microbiol., 94, 230-239 (2003).
- Dagher F., Deziel E., Lirette P., Paquette G., Bisaillon J. G. and Villemur R., Can. J. Microbiol., 43, 368-377 (1997).
- McLellan S. L., Warshawsky D. and Shann J. R., Environ. Toxicol. Chem., 21, 253-259 (2002).
- Toledo F. L., Calvo C., Rodelas B. and Gonzalez-Lopez J., Syst. Appl. Microbiol., 29, 244-252 (2006).
- Throne-Holst M., Wentzel A., Ellingsen T. E., Kotlar H. K. and Zotchev S. B., Appl. Environ. Microbiol., 73, 3327-3332 (2007).
- Makula R. A., Lockwood P. J. and Finnerty W. R., J. Bacteriol., 121, 250-258 (1975).
- Rosenberg M., Gutnick D. and Rosenberg E., FEMS Microbiol. Lett., 9, 29-33 (1980).
- Asperger O., Naumann A. and Kleber H. P., FEMS Microbiol. Lett., 11, 309-312 (1981).
- Sakai Y., Maeng J. H., Tani Y. and Kato N., Biosci. Biotechnol. Biochem., 58, 2128-2130 (1994).
- Throne-Holst M., Markussen S., Winnberg A., Ellingsen T. E., Kotlar H. K. and Zotchev S. B., Appl. Microbiol. Biotechnol., 72, 353-360 (2006).
- Briganti F., Pessione E., Giunta C. and Scozzafava A., FEBS Lett., 416, 61-64 (1997).
- Abdel-El-Haleem D., Moawad H., Zaki E. and Zaki S., Microb. Ecol., 43, 217-224 (2002a).
- Zilli M., Palazzi E., Sene L., Converti A. and Borghi M. D., Process. Biochem., 10, 423-429 (2001).
- Soojhawon I., Lokhande P. D., Kodam K. M. and Gawai K. R., Enzyme and Microbial Technology, 37, 527-533 (2005).
- Auling G., Pilz F., Busse H. J., Karrasch S., Streichan M. and Schon G., Appl. Environ. Microbiol., 57, 3585-3592 (1991) .
- Wagner M., Erhart R., Manz W., Amann R., Lemmer H., Weidi D. and Schleifer K. H., Appl. Environ. Microbiol., 56, 3125-3129 (1994).
- Boswell C. D., Dick R. E., Essles H. and Macaskie L. E., J. Ind. Microbiol. Biotechnol., 26, 333-340 (2001).
- Verma S., Bhargava R. and Pruthi V., International Biodeterioration & Biodegradation, 57, 207-213 (2006).
- Abdel-El-Haleem D., Afr. J. Biotechnol., 2, 71-74 (2003).
- Carson C. A., Shear B. L., Ellersieck M. R. and Asfaw A., Appl. Environ. Microbiol., 67, 1503-1507 (2001).
- Rappe M. S., Connon S. A., Vergin K. L. and Giovannoni S. J., Nature, 418, 630-633 (2002).

This work is licensed under a Creative Commons Attribution 4.0 International License.





