Manuscript accepted on : February 17, 2009
Published online on: 28-06-2009
B. S. Chaithra1, Y. Hari Babu1, S. Venkateshwar2 M. Narsi Reddy2*
1Department of Veterinary Microbiology, Veterinary College, Bidar - 585 401 India.
2Centre for Biotechnology, University College of Technology, Osmania University, Hyderabad - 500 007 India.
Corresponding Author E-mail: mnreddydr@gmail.com
ABSTRACT: Bacteriophage to an Escherichia coli isolate that is pathogenic in poultry was isolated from faecal samples of chickens. A study was conducted to evaluate the therapeutic efficacy of bacteriophage and the antibiotic enrofloxacin individually and in combination to treat colibacillosis.The experimental design was made with ten chickens each in five groups. The group I as control, unchallenged with E.coli, group II as challenged with E. coli, group III with E.coli and bacteriophage inoculations, group IV with E.coli and antibiotic (enrofloxacin), while the group V with combination of E.coli, bacteriophage and enrofloxacin. Birds in the group II were challenged at 7th day of age by injecting 105 cfu/ml of E.coli into the thoracic air sac. The antibiotic treatment was initiated immediately after the birds were challenged and consisted of 50 ppm enrofloxacin in the drinking water for 7 consecutive days. The bacteriophage treatment consisted of intramuscular injection of bacteriophage (1010pfu/ml) for 3 days administered immediately after the E.coli challenge. Mortality in the birds challenged with E.coli and untreated was 60% and the bacteriophage treatment significantly decreased mortality to 10 %. In the antibiotic treatment group morbidity was seen in only one bird and there was no mortality. The results indicated the effectiveness of bacteriophage treatment. Though the combination of bacteriophage with antibiotic (enrofloxacin) showed equal results, it was suggested that better usage of bacteriophage in treating colibacillosis as it lacks side effects that regularly been envisaged through the antibiotic therapy.
KEYWORDS: E.coli; Bacteriophage; enrofloxacin; poultry; antibiotic therapy
Download this article as:| Copy the following to cite this article: Chaithra B. S. Babu Y. H, Venkateshwar S, Reddy M. N. Study on the Therapeutic Efficacy of Isolated Bacteriophage on Experimentally Induced Escherichia Coli Infection in Chickens. Biosci Biotechnol Res Asia 2009;6(1) |
| Copy the following to cite this URL: Chaithra B. S. Babu Y. H, Venkateshwar S, Reddy M. N. Study on the Therapeutic Efficacy of Isolated Bacteriophage on Experimentally Induced Escherichia Coli Infection in Chickens. Biosci Biotechnol Res Asia 2009;6(1). Available from: https://www.biotech-asia.org/?p=8170 |
Introduction
The emergence of pathogenic bacteria with resistance to antibiotics has reduced the effectiveness of antibiotic treatment of infections. As cases of infections caused by antibiotic resistant bacteria increases there is growing concern over the use of antibiotics in animal medicine.
Colibacillosis is considered as the most common cause of death in the poultry industry necessitating the use of antibiotics targeted at E.coli.Because of the antibiotic resistance there is a real need to use alternatives to antibiotics for both the prevention and treatment of E.coli infection in poultry.
Bacteriophages are viruses that can kill bacteria and it is possible to use these viruses to treat bacterial diseases. These bacteriophages were co discovered in the early 1900s by Twort (1915) and d’Herelle (1917).The use of bacteriophages for biological control of pathogens of poultry has aroused much interest in recent years since; no drug residues and drug toxicity are associated with this type of therapy (Wu and Chao, 1982).Due to these reasons, bacteriophage therapy could be an attractive adjunct and viable proposition for controlling bacterial infections than other control methods in poultry farming.
Objectives of this study were to isolate bacteriohage to an E.coli strain pathogenic in poultry and to compare the therapeutic efficacy of isolated bacteriophage vis-à-vis antibiotic.
Material and Methods
Bacteriophage isolation
Faecal samples from chickens were collected from poultry farms in normal saline. The sample was centrifuged at 10000g for 10 min.One ml of the supernatant was added to five ml of 2.5-3 hrs old E.coli culture grown on Luria Berteni broth, the mixture was incubated for 3 hrs at 370C.After incubation the tubes were centrifuged at 10000g for 10 min.Bacteriophages were isolated from these enrichment tubes by serially diluting the supernatant and preparing the soft agar overlay plates. The Luria Berteni soft agar was prepared to a final concentration of 0.3% agar, and 0.1% of Magnesium chloride. The procedure was to pre incubate Luria Berteni agar plates at 450C to dry the agar surfaces. Next one ml of 2.5hrs E.coli stock culture was added as was 0.1 ml of the enrichment supernatant to 5 ml of Luria Berteni soft agar tubes. The tubes were mixed and then poured over the Luria Berteni agar plates. The plates were incubated at 370C overnight. Clear zones representing bacteriophage plaques were apparent. With these procedures we isolated two bacteriophages.
Experimental design of the study
A study was conducted to determine the efficacy of treating Colibacillosis with a bacteriophage, antibiotic-Enrofloxacin or a combination of the bacteriophage and antibiotic-Enrofloxacin. A total of 50 chicks were obtained and maintained for 3 weeks of age. Chicks were divided into 5 groups. Experimental design of the study on chickens is furnished in Table 1. Chicks in the I group were kept as unchallenged control i.e., birds that were not challenged with Escherichia coli. Chicks in the II, III, IV and V groups were challenged with Escherichia coli at 7th day of age by injecting 0.1 ml of a 2.5 hrs culture of E.Coli containing 105 cfu/ml into the thorasic air sac. Chicks in the III group were treated with 1010 pfu/ml of bacteriophage intra muscularly at 7th day of age for 3 consecutive days. Chicks in the IV group were treated with 50 ppm enrofloxacin through drinking water at 7th day of age for 7 consecutive days. Chicks in the V group were treated with both 1010 pfu/ml of bacteriophage and 50 ppm enrofloxacin.The birds were observed for the appearance of clinical signs and morbidity and mortality was recorded.
Table1. Experimental design of the study on chickens.
| Groups | Unchallenged control | E.coli
Challenged |
Bacteriophage treatment | Antibiotic treatment | Phage +Antibiotic treatment |
| 1st | + | – | – | – | – |
| 2nd | – | + | – | – | – |
| 3rd | – | + | + | – | – |
| 4th | – | + | – | + | – |
| 5th | – | + | – | – | + |
Results
Isolation of bacteriophages
Two types of phages were recovered from faecal samples of chickens. Both types of phages were producing small plaques measuring about 1-1.5mm in diameter. As for as their size was concerened, both had round edges. The phage lysates were separately preserved in broth at -200C for one month and found active equally and there was no significant decrease in their efficiency of plating.
 |
Figure 1: Plaques formed by the lysis of E.coli by bacteriophage.
|
Experimental study on chickens
The I group (unchallenged control) neither showed the signs of disease nor mortality. The II group (challenged control) showed the signs of illness after 18 hrs.They became lethargic, standing with dropping wings and eventually collapsed. All 10 birds showed morbidity while mortality occurred in six birds (60%) after 24 hrs.The III group (Bacteriophage treatment) showed mortality in only one bird (10%) after 48hrs and there was no morbidity in remaining birds. In the IV group (Antibiotic treatment) morbidity was seen in only one bird and there was no mortality. In the V group (Bacteriophage + Antibiotic treatment) no morbidity and mortality were seen. The detailed results are shown in table 2.
Table 2: In vivo Bacteriophage activity against pathogenic E.coli in chicken.
| Group | No. of birds | Morbidity | Mortality |
| Unchallenged control (I) | 10 | 0 | 0 |
| Challenged control(II) | 10 | 10 | 6 |
| Bacteriophage treatment(III) | 10 | 1 | 1 |
| Antibiotic treatment(IV) | 10 | 1 | 0 |
| Bacteriophage + Anti biotic treatment(V) | 10 | 0 | 0 |
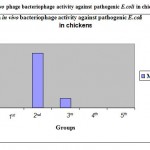 |
Figure 2: An invivo phage bacteriophage activity against pathogenic E.coli in chickens.
|
Discussion
The results of the present study indicated the effectiveness of bacteriophage treatment where the mortality was 10% only and which is negligible. Though the combination of bacteriophage with antibiotic showed equal results, it is suggested that better usage of bacteriophage in the treatment of colibacillosis,as it lacks side effects that is regularly been envisaged through the antibiotic therapy. These results are comparable with those observed by Tanji and colleagues (2005) who reported the therapeutic use of phage for controlling E.coli O157:H7, which was associated with hemorrhagic colitis. Barrow et al., (1998) reported significant protection in chickens from an intramuscular injection of E. coli when simultaneously injected at a different site with a bacteriophage, and some protection when E. coli was injected intracranial and the bacteriophage was simultaneously injected intramuscularly.
Although antibiotic therapy is thought to be the most effective therapy against infections, such therapy is frequently ineffective due to bacterial drug resistance. Bacteria become less susceptible to antibiotic treatment by allowing the bacteria to generate offspring in subsequent generations that develop a stronger resistance to the antibiotic. Their resistance is due to the minute population of bacteria that survive the antibiotic treatment reproducing with mutations beneficial to the survival of the bacteria in the antibiotic condition (Tanji et al.,2005).Whereas, the rate of developing resistance to phages is approximately 10-fold lower than that to antibiotics. Cocktails of several phages can be used to get sufficient breadth of host range and to reduce the probability of resistance developing. (Carlton, 1999). Due to the malleable genetic character of E.coli, it has one of the widest spectra of disease of any bacterial species (Donnenberg et al.,2002).Antibiotic use in E.coli infections is of doubtful value since resistance is wide spread in E.coli,and vaccines are still in the early development phase (Sevarine et al.,2002). Bacteriophages attack specifically bacterial cells and eventually eliminate the whole population (Wong and Loung, 2000).Tanji et al., (2005) suggested that antibiotics usually affect not only the targeted pathogenic bacteria, but also normal microbial balance, which may lead to serious secondary effect including intestinal disorder. The development costs of phage therapy are much lower than for a new antibiotic. Therapeutic phage production could be carried out without biohazard containment conditions and in cheap media, providing an affordable technology even where the burden of E.coli infection is the highest (Chibani-Chennoufi et al., 2004).The chances of developing a successful phage approach to control E.coli infections are reasonably good since it can be based on decades of research with the bacterium and its phages.
References
- BARROW, P., LOVELL, M. AND BERCHIERI, J.R., (1998). Use of lytic Bacteriophage for control of experimental Escherichia coli septicemia and meningitis in chickens and calves. Clin. Diagn. Lab. Immunol. 5: 294–298.
- CARLTON, R. M., (1999). Phage therapy: past history and future prospects. Arch. Immunol. Ther. Exp. 5:267-274.
- CHIBANI-CHENNOUFI, S., SIDOTI, J., BRUTTIN, A., KUTTER, E., SARKER, S. & BRUSSOW, H., (2004). In vitro and in vivo bacteriolytic activities of Escherichia coli phages: implications for phage therapy. Antimicrob Agents Chemother 48: 2558–2569.
- D’HERELLE, F., (1917). Sur un microbe invisible antagoniste des bacilles dusenteriques. C. R. Acad. Sci., (Paris) 165: 373-375.
- DONNENBERG, M. S. (2002). Escherichia coli: Virulence Mechanisms of a Versatile Pathogen. Amsterdam press.
- SEVARINE, S. J., HALL, E. R., BASSILY, S.,(2002).Introductory evaluation of an oral, killed whole cell enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Egyptian infants. Pediatr Infect Dis J. 21:322–330.
- TANJI, Y., K. MIZOGUCHI, M. YOICHI, M. MORITA, N. KIJIMA, H. KATOR, AND H. UNNO., (2005).Therapeutic use of phage cocktail for controlling Escherichia coli O157:H7 in gastrointestinal tract of mice.J.biosci.and bioengg.100 : 280-287.
- TWORT, F. W., (1915). An investigation on the nature of ultramicroscopic viruses. Lancet, 11: 1241.
- WANG, L.M., LOUNG, R., (2000).Phage will out. Strategies of host cell lysis.Trends Microbiol, 8:120-128.
- WU, J. L. and CHAO, W. J., (1982). Isolation and application of a new bacteriophage, jET-1, which infect Edwardsiella tarda, the pathogen of Edwardsiellosis. CAPD Fisheries Series No. 8, Fish Dis. Res., (IV), pp 8-17.

This work is licensed under a Creative Commons Attribution 4.0 International License.





