How to Cite | Publication History | PlumX Article Matrix
Antihyperlipidemic Activity of Methanolic Extract of Lagenaria Siceraria
Vasu Keshetty1, Srinivas Pabba1, Puligilla Shankaraiah2 and Allenki Venkatesham1*
1SVS institute of Pharmacy, Ramaram, Warangal - 506 015 India.
2Department of Pharmacy, Kakatiya University, Warangal - 506 009 India.
Corresponding Author E-mail: venkatkuc@gmail.com
ABSTRACT: The present study is designed to evaluate the effect of methanolic extracts of Lagenaria siceraria on lipid profiles in Triton X-100 induced hyperlipidemia in male wistar rats. The fruit juice was obtained by crushing the fresh fruits of L. siceraria in the juicer and was subsequently dried in the oven at 40°-50°C. The parent dried juice extract was then fractionated by using the solvents according to polarity in ascending order i.e. by using chloroform: acetic acid, methanol, pyridine, and water. Each fraction was dried in oven at 40°-50°C. Thin layer chromatography (TLC) used active fraction obtained by column chromatography for further isolation and the isolated compounds (LS1, LS2 and LS3) were tested for anti-hyperlipidemic activity. All the extracts significantly increased (p < 0.0001) plasma HDL-Cholesterol and decreased plasma TC, LDL-Cholesterol and TG levels as compared with hyperlipidemic control.
KEYWORDS: Lagenaria siceraria; lipid profile; Triton X-100; anti-hyperlipidemic
Download this article as:| Copy the following to cite this article: Keshetty V, Pabba S, Shankaraiah P, Venkatesham A. Antihyperlipidemic Activity of Methanolic Extract of Lagenaria Siceraria. Biosci Biotechnol Res Asia 2009;6(1) |
| Copy the following to cite this URL: Keshetty V, Pabba S, Shankaraiah P, Venkatesham A. Antihyperlipidemic Activity of Methanolic Extract of Lagenaria Siceraria. Biosci Biotechnol Res Asia 2009;6(1). Available from: https://www.biotech-asia.org/?p=8197 |
Introduction
Atherosclerosis remains the major cause of death and premature disability. Hyperlipidemia is the most firmly established and best understood risk factor for atherosclerosis1. Despite of differences in lipoprotein distribution and metabolism between humans and rats, hyperlipidemic rat models are extensively used in lipid research2. Condiments, medicinal plants, fruits used in day to day preparation of Indian kitchens have been identified as hypolipidaemic in ayurveda. In the present study, Lagenaria siceraria (LS) was selected for the evaluation of antihyperlipidemic activity in rats. The plant, Lagenaria siceraria (Mol) Standl. (Family: Cucurbitaceae) is commonly known as bottle gourd, is a common fruit vegetable in India. It is an excellent fruit in the nature having composition of all the essential constituents that are required for normal and good health of humans3. L. siceraria fruits are traditionally used for its cardioprotective, cardiotonic, general tonic, aphrodisiac and acts as alternate purgative, diuretic agent 4. The fruit juice extract was used as an analgesic and anti-Inflammatory activities5. The present study investigated the antihyperlipidemic activity of methanolic extract of Lagenaria siceraria in Triton X-100 induced hyperlipidemic rats.
Materials and Methods
Extraction of plant material
The fresh fruits of Lagenaria siceraria (LS) were obtained in the months of August-December, from the local market of Hyderabad, India and it was taxonomically identified and authenticated as Lagenaria siceraria Linn. by Prof. V. S. Raju, Dept of Botany, Kakatiya University, Warangal, India. The fruit juice of L. siceraria was obtained by crushing the fresh fruits in the mixer and subsequently dried in the oven at 40-50°C.
Preliminary phytochemical screening
LSFJE was studied for its preliminary phytochemical screening 6 for the detection of various plant constituents.
Fractional extraction of parent extract
The parent dried juice extract was then fractionated by using the solvents according to polarity in ascending order i.e. by using chloroform: acetic acid, methanol, pyridine and water. Each fraction was dried in oven at 40-50C.
Column Chromatographic Isolation and Purification of Methanolic Extract
Column: Glass
Dimension: Length 50 cm, diameter 3.5 cm
Stationary phase: Silica gel
Sample: Methanolic ether extract
Mobile phase: Gradient elution
The Methanolic fraction of parent extract was subjected for isolation over the silica gel (mesh size- 100-250) column. Previously, the slurry of silica gel was prepared with the mobile phase. The column was washed with the mobile phase for sufficient period of time. Then Methanolic fraction was loaded over the silica gel. The mobile phase was passed continuously with constant flow rate (10 ml/min.). The fractions were collected at regular intervals of time, evaporated at temperature <40-50°C and subjected for the evaluation of anti-hyperlipidemic activity in Triton-X-100 induced hyperlipidemia in rats.
Isolation of compounds using Thin Layer Chromatography (TLC)
Active fraction obtained by column chromatography was used for further isolation by TLC. The solvent system developed on trial and error basis was n-butanol: methanol: water (6:2:2). Four spots were obtained and were named as LS1, LS2, and LS3. The isolated spots were collected by using preparative Thin Layer Chromatography.
Chemicals
Atorvastatin pure drug was a kind gift from Dr. Reddy’s Pharmaceutical Ltd, Hyderabad. All other chemical used were analytical grade from SD fine chem., India. Cholesterol kit (Enzymatic Method) and HDL-C kit were procured from Qualigens Diagnostics, Mumbai. Triglycerides kit was obtained from E-Merck Limited, Mumbai, India.
Animals
Male Wister albino adult rats weighing 200-220g were selected and housed in polypropylene cages in a room where the congenial temperature was 27°C ±1°C and 12 hrs light and dark cycles were maintained. The animals were allowed to acclimatize to the environment for 7 days and supplied with a standard pellet diet (Hindustan Lever Ltd., Bangalore) and water ad libitum. Hyperlipidemia was induced in rats by single intraperitoneal injection of freshly prepared solution of Triton-X-100 (100 mg/kg) in physiological saline after overnight fasting for 18 hours 7,8.
Anti–hyperlipidemic studies
The rats were divided into five groups of eight rats in each group and were treated with single dose/day (p.o) of standard drug or extracts of LS.
Group I: (Control) Received normal saline (p.o).
Group II: (HL) Rats were treated Triton-X-100 (100mg/kg, i. p).
Group III: Rats were treated Triton-X-100 (100mg/kg, i. p) + LS 1(10 mg/kg, p.o).
Group IV: Rats were treated Triton-X-100 (100mg/kg, i. p) + LS 2(10 mg/kg, p.o).
Group V: Rats were treated Triton-X-100 (100mg/kg, i. p) + LS 3(10 mg/kg, p.o).
Group VI: (AT) Treated with Triton and Atorvastatin (10 mg/kg. p.o).
The protocol of the present study was approved by the Institutional Animal Ethics Committee.
Collection of blood samples
The blood was collected by retro orbital sinus puncture, under mild ether anesthesia in heparinized tubes. Serum obtained by immediate centrifugation of blood samples using remi ultra cooling centrifuge at 3000 rpm for 5 minutes at room temperature and was directly used for estimating serum lipid profiles (serum TC, TG, LDL-C and HDL-C). All samples were stored at 4°C until analysis.
Biochemical analysis
Plasma lipid levels include TC, TG and HDL-C were carried out using respective diagnostic commercial kits 9 from Qualigens diagnostics, Mumbai, India and LDL-C in plasma was calculated as per friedewald estimation 10. LDL-C= (TC-(TG/5+HDL)-C) mg/dl
Statistical Analysis
The results were expressed as mean ± SD. The Triton control was compared with normal and the experimental results were compared with Triton control. Statistical analysis was carried out using paired t-test and one-way ANOVA followed by Bonferroni’s test. Differences below P<0.05 implied statistically significance.
Results and Discussion
Preliminary phytochemical screening
Preliminary phytochemical screening of the methanolic extract of Lagenaria siceraria showed the presence of flavonoid glycosides, cucurbitacin saponins, proteins and carbohydrates.
Natural remedies have been investigated for centuries for a wide variety of ailments. Lagenaria siceraria has been used traditionally as a cardiprotective, cardiotonic, aphrodiasic and diuretic agent 4. In the present study, Lagenaria siceraria was selected to screen for it antihyperlipidemic activity in Triton X-100 (100 mg/kg) induced hyperlipidemic rats.As reported earlier, Injection of Triton X-100 (100 mg/kg) has successfully induced hyperlipidemia in rats by increasing the serum TC, TG and LDL-C levels. The effect of methanolic extracts of Lagenaria siceraria on serum lipid profile levels was showed in Table 1. Treatment with methanolic extracts (LS 1, LS 2 and LS 3) of L. siceraria at the doses of 10mg/kg significantly (P < 0.0001) reduced the serum TC, TG and LDL-C levels and increased the serum HDL-C levels when compared to the hyperlipidemic (HL) control group. The change in lipid levels in groups of II, III and IV were comparable with group of atrovastatin treated rats. Among three fractions, LS1 reduced the elevated lipid levels more significantly than the others. This study exhibited the elevated blood cholesterol, triglycerides and LDL-C, which occurs in hyperlipidemia, was significantly reduced by the oral administration of all L. siceraria extracts in rats. This finding provides some biochemical basis for the use of fruits, fruit juice in the management of hyperlipidemia.
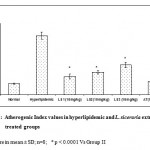 |
Figure 1: Atherogenic Index values in hyperlipidemic and L. siceraria extracts treated groups.
|
Values are in mean ± SD; n=8; * p < 0.0001 Vs Group II
Table 1: Effect of methanolic extracts of L. siceraria on serum lipid profile levels in. Triton induced hyperlipidemic rats.
|
GROUP |
TC
(mg/dl) |
TG
(mg/dl) |
HDL-C
(mg/dl) |
LDL-C (mg/dl) |
| Group I: Normal | 83.51+ 1.87 | 69.07 + 1.93 | 43.73 +1.47 | 75.51+ 2.24 |
| Group II: HL(Triton) | 146.51 + 3.85b | 156.20 +5.70b | 24.66+0.19b | 198.73+5.23b |
| Group III: Triton +
LS 1 (10 mg/kg) |
130.62 + 3.55 a |
112.73 +4.09 a |
54.43 +1.71 a |
131.26+6.92 a |
| Group IV: Triton +
LS 2 (10 mg/kg) |
131.22 + 3.16 a |
120.11 +4.07 a |
47.41 +2.12 a |
149.92+1.91 a |
| Group V: Triton +
LS 3 (10 mg/kg) |
134.24 + 4.51 a |
124.61 +3.22 a |
36.14+1.57 a |
152.27+6.19 a |
| Group VI: Triton +AT
Atorvastatin(10mg/kg) |
108.45 + 5.13 a |
89.24 + 5.64 a |
50.44 +5.54 a |
112.67+4.16 a |
Values are in mean ± SD; n=8; a= p < 0.0001 Vs Group II; b= p < 0.0001 Vs Group I
It is widely accepted that reduction in plasma HDL is a risk factor for developing atherosclerosis. HDL facilitates the translocation of cholesterol from the peripheral tissue, such as arterial walls to liver for catabolism. The increase in HDL may slow down the atherosclerotic process11. Increased levels of HDL (cardio protective lipid) after administration of L. siceraria extracts concluded that the extract is a potent cardio protective agent. High levels of TC and LDL-C are major coronary risk factors12, 13. Administration of MELS lowered both total and LDL cholesterol in hyperlipidemic rats. This lowering of TC and LDL-cholesterol would reduce the incidence of coronary events 14Atherogenic Index (AI)
Atherogenic Index was calculated by the equation: (total cholesterol-HDL-cholesterol)/ HDL-cholesterol14. The values of AI in hyperlipidemic and methanolic extracts of LS treated groups were represented in figure 1. The ratio was significantly increased in Triton induced hyperlipidemic rats compared with normal group and these elevated ratios were returned to near normal levels in groups of rats treated with methanolic extracts of L. siceraria and atorvastatin. The rise in AI in hyperlipidemic rats enhances the probability of cardiovascular pathogenesis and endothelial dysfunction. A significant decrease in AI value was observed in herbal supplemented animals, suggests the atheroprotective / cardio protective potential of this herb. The present investigation concluded, all the fractions obtained from the methanolic extracts of L. siceraria significantly reduced the Triton-X-100 induced hyperlipidemia in rats.
References
- Libby P. Prevention and treatment of atherosclerosis. In Harrison‘s principles of internal medicine, McGraw Hill; pp. 1430 (2005).
- Raasch RH. Hyperlipidemia. In: L.Y. Young, M.A. Koda-Kimble (Eds), Applied Therapeutics. The Clinical Use of Drugs, Edwards Brothers, Ann Arbor, MI, pp. 1743 (1988).
- Shirwaikar A, Sreenivasan KK. Ind J Pharm Sci., 58: 197(1996).
- Nadkarni KM, Nadkarni AK. Indian Meteria Medica, Vol 1, Popular Prakashan, New Delhi, pp. 722(1996).
- Ghule BV, Ghante MH, Upaganlawar AB, Yeole PG. Pharmacognosy Magazine. 8: 232(2006).
- Kokate CK. Practical Pharmacognosy, Vallabh Prakashan, New Delhi, pp. 107 (1994).
- Moss JN, Dajani EZ.Antihyperlipidemic agents. In: Turner RA, Hebben PA. Screening methods in Pharmacology. Vol 2. Academic Press. New York: pp. 121 (1971).
- Vogel G, Vogel WH. Influence of lipid metabolism. In: Drug Discovery and Evaluation pharmacological assay. Springer-Verly, Berloin; pp. 604(1997).
- Gulati R, Agarwal DK, Hossain MM, Ali R, Srivastava U. Indian J. Physiol Pharmacol; 47:357(2003)
- Friedewald VT, Levy RI, Fredrickson DS. Clin Chem; 18:499(1972).
- Nofer JR, Kehrel B, Fobker M, Levkau B, Assmann G, Eckardstein AV. Atherosclerosis; 161:1(2002).
- Temme EH, Van HPG, Schouten EG, Kesteloot H. Acta Cardiology; 57: 111 (2002).
- National cholesterol Education Program Expert Panel. Circulation; 89:1450 (1994)
- Lipid Research Clinic Program. Journal of American Medical Association; 215:25 (1984a).

This work is licensed under a Creative Commons Attribution 4.0 International License.





