How to Cite | Publication History | PlumX Article Matrix
Varahala Rao Vadlapudi1 and Varaprasad Bobbarala2
1Department of Botany, Andhra University, Visakhapatnam India
2For U Biosciences, A/4A, Park lane Residency, East Point Colony, Visakhapatnam - 530 017 India.
ABSTRACT: Antimicrobial effect of the crude methanolic extracts of Hibiscus tiliaceous and Aegiceras corniculatum was studied. Agar well diffusion method has been adopted in this study and petri dishes containing nutrient and potato dextrose agar medium seeded with the test microorganism belonging to plants and clinical were used for antimicrobial screening. Test materials diffuse from the wells to the surrounding medium of the plate. The plates are then kept in an incubator (37°) for 18 hours to allow the growth of the microorganisms. The antibacterial activity of the test agent is determined by measuring the diameter of the zone of inhibition in term of millimeter. Antimicrobial screening showed that the crude methanol extract at concentration at 100mg/ml possess antimicrobial activity against most of the test organisms depending upon the nature of their active ingredients in the extract and capacity of diffusion into the agar medium. Among the test organisms, the extract showed significant antimicrobial activity against Cladosporium herbarum, Erwinia carotovora and Streptococcus salivarius, whereas Ustilago maydis and Candida albicans showed no activity. The present study was conducted to develop newer lead for better and safer chemotherapeutic agents from natural resources
KEYWORDS: Antimicrobial; Methanol extracts; Hibiscus tiliaceous; Aegiceras corniculatum; Zone of inhibition
Download this article as:| Copy the following to cite this article: Krothapalli U, Nuthi L. S. B, Kumar V. P. Wheat and Salanity: Response of Different Concentrations of Nacl and Kcl. Biosci Biotechnol Res Asia 2008;6(1) |
| Copy the following to cite this URL: Krothapalli U, Nuthi L. S. B, Kumar V. P. Wheat and Salanity: Response of Different Concentrations of Nacl and Kcl. Biosci Biotechnol Res Asia 2008;6(1). Available from: https://www.biotech-asia.org/?p=8220 |
Introduction
Hibiscus tiliaceous L. belongs to Malvaceae has many traditional uses around the world including to cool fevers and soothe coughs (leaves), treat dysentery (bark), ear infections and abscesses (flowers), as laxative (bark and flower). Aegiceras corniculatum L. Blanco is commonly known as r iver mangrove belongs to family myrsinaceae and vernacular name is guggilam are used in the treatment of different diseases, stem extracts for treatment of oral cancer (Roome et al., 2008) and Leaf decoction applied sore ears.
The control of microbial infections has been remarkably effective since the discovery of antifungal and antibacterial drugs. However some
of the pathogens rapidly become resistant to many of the first discovered effective dr ugs. The development of drug resistance as well as appearance of undesirable side effects of certain antibiotics (WHO, 2002) has led to the search of new antibacterial agents in particular from medicinal plants. Plant materials continue to play a major role in primary health care as therapeutic remedies in many developing countries (Zakaria, 1991). Higher plants have been shown to be a potential source for new anti-microbial agents (Mitscher et al., 1987). Kokpal et al., (1990) had also reported the bioactive compounds from mangrove plants Combs & Anderson (1949) have reported the presence of compounds like tannins, alkaloids and polyphenols in mangroves which play an important role in the suppression of deleterious microorganisms (Jamale 322 Vadlapudi & Bobbarala, Biosci., Biotech. Res. Asia, Vol. 6(1), 321-324 (2009) & Joshi 1998; Nishiyama et al., 1978; Ross et al., 1980). The study of Premnathan et al., (1992 and 1996) revealed that the mangroves were found highly effective for antiviral activity as compared to seaweeds and sea grasses. So in this view screening of mangrove plant extracts has been of great interest to scientist for the discovery of new drugs effective in the treatment of several diseases (Dimayuga and Garcia, 1991). As a part of our study for the search of bioactive secondary metabolites from medicinal plants we have investigated methanolic extract of H. tiliaceous and corniculatum for potential antimicrobial activity. A preliminary screen revealed that the crude and partially extracted fraction markedly inhibit the growth of the microorganisms. The goal of this study was to increase the knowledge of the antimicrobial activity of mangrove plants.
Material and Methods
Mangrove plants A. corniculatum and H. tiliaceous growing in the saline intertidal zones of sheltered coast lines. It has been reported to tolerate extreme weather conditions, high winds. The plant par ts were collected from Coringa Mangrove Wetland, Andhra Pradesh, India. The plant material was taxonomically identified and the Voucher specimen is stored. The plant material were dried under shade with occasional shifting and then
powdered with a mechanical grinder and stored in an airtight container. The powder obtained was subjected to successive soxhlet extraction with the organic solvents with increasing order of polarity.
The antimicrobial activity of the methanolic extracts was assessed against microbial strains of clinical, and plant origin these strains include Bipolaris bicolor (MTCC 2105), Candida albicans (MTCC 3017), Cladosporium herbarum (MTCC 2143), Curvularia lunata (MTCC 2030), Erwinia carotovora (MTCC 3609), Pseudomaonas marginales (MTCC 2758), Pseudomonas syringae (MTCC 1604), Staphylococcus aureus (MTCC 96), Streptococcus anginosus (MTCC 1929), Streptococcus gordonii (MTCC 2695), Streptococcus mitis (MTCC 2696), Streptococcus mutans (MTCC 497), Streptococcus salivarius (MTCC 1938), Ustilago maydis (MTCC 1474) and Xanthomonas campestris (MTCC 2286) were obtained from Microbial Type Culture Collection (MTCC), Chandigarh were used as test organisms. The strains are maintained and tested on Nutrient Agar (NA) for bacteria and Potato Dextrose Agar (PDA) for fungi.
Determination of antibacterial activity
The antimicrobial activity of the hexane, chloroform, methanol and water extracts of each sample was evaluated by using well-diffusion
Volume per well: 50µl; Borer size used: 6mm; Extract concentration in 100mg/ml
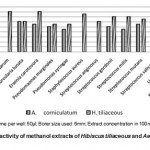 |
Figure 1: Antimicrobial activity of methanol extracts of Hibiscus tiliaceous and Aegiceras corniculatum.
|
Vadlapudi & Bobbarala, Biosci., Biotech. Res. Asia, Vol. 6(1), 321-324 (2009) 323 method or cup plate method of Murray et al., (1995) modified by Olurinola (1996). 20 ml of nutrient agar was dispensed into sterile universal bottles these were then inoculated with 0.2 ml of cultures mixed gently and poured into sterile petridishes. After setting a number 3-cup borer (6mm) diameter was properly sterilized by flaming and used to make three to five uniform cups/wells in each petridish. A drop of molten nutrient agar was used to seal the base of each cup. The cups/wells were filled with 50 µl of the different extracts of 100mg/ml and allow diffusing for 45minutes. The solvents used for reconstituting the extracts were similar ly analyzed. The plates w ere incubated at 37°c for 24hours . The standard antibiotic drugs was used at different concentrations to get MIC (Minimum inhibitory concentrations) the antibiotic drug used were Streptomycin. The zones of inhibition were measured with antibiotic zone scale in mm and the experiment was carried out in triplicates.
Results
The antimicrobial activity expressed in term of diameter of zone of inhibition in millimeter.
H. tiliaceous shown highest activity against herbarum and E. carotovora followed by C. albicans and etc. and no activity against U. maydis whereas
A. corniculatum extract showed highest activity against U. maydis and no activity with C. herbar Hexane and chloroform extracts shown no activity hence results are not presented.
Discussion
The methanolic extracts of H. tiliaceous shown highest activity (16 mm) and A. corniculatum have no activity on C. herbarum. U. maydis has no activity with H. tiliaceous but with A. corniculatum. The hexane and chloroform extract appears to have less antibacterial and antifungal activity than the methanolic extracts. The antimicrobial activity of these cr ude extracts due to the secondar y metabolites or compounds e.g. glycoside, saponin, tannin, flavonoids, terpenoid, alkaloids, conferred by many researchers (Okeke et al., 2001; Ebi and Ofoefule et al., 1997). The variation of antimicrobial activity among the extracts tested is due to distribution of antimicrobial substances, which varied from fraction to fraction of the crude extract. The present study was conducted to develop newer lead for better and safer chemotherapeutic agents from Kakinada and Godavari. Further studies are needed to identify the pure component and establish the exact mechanism of action for antibacterial action of the plant extract.
It can be concluded that plant extracts have greater potential as antimicrobial compounds against micro flora and that they can be used in the treatment of infectious diseases caused by resistant pathogenic microorganisms.
References
- Combs C.A. and H. , Use of mangrove bark, Australian leather trade Rev., 43: 270-274 (1949).
- Dimayuga E. and Garcia S.K., Antimicrobial screening of medicinal plants from Baja California Sur.Mexico. Journal of Ethnopharmacology., 31: 181-192 (1991).
- Ebi C and Ofoefule S.I., Investigating into folkloric antimicrobial activities of Landolphia owerrience. Phytotherapy Research., 11: 149-151 (1997).
- Jamale B.B. and V. Joshi., Effect on age of mineral constituents Polyphenoloxides and peroxides in mangrove leaves, In. J. Exp. Biol., 16(1): 117-120 (1998).
- Kokpal V. Miles D.H. Payne M and Chittawong V., Chemical constituents and bioactive compounds from mangrove plants, Studies in Natural Products Chemistry 7: 175-199 (1990).
- Mitscher A. Drake S. Gollopudi S.R. and Okwute S.K., A modern look at folkloric use 324 Vadlapudi & Bobbarala, Biosci., Biotech. Res. Asia, Vol. 6(1), 321-324 (2009) of anti-infective agents. Journal of Natural Products, 50: 1025-1040 (1987).
- Murray P.R. Baron J. Pfaller M.A. Tenover F.C. and Yolken H.R., Manual of Clinical Microbiology, 6th Edition. ASM Press, Washington. DC, 15-18 (1995).
- Nishiyama Y. Ryuzo P.C. Sanchez and Kozaki. Inhibitory functions of Mangrove bark towards cell growth of microorganisms. Hakko, Kogaku, Kaishi, 56: 712-717 (1978).
- Okeke I. Iroegbu C.U. Eze E.N. Okoli A.S. and Esimone C.O., Evaluation of extracts the root of Landolphia owerr ience for antibacter ial activity. Jour nal of the Ethnopharmacology, 78, 119-127 (2001).
- Olur inola P.F., A laborator y manual of pharmaceutical microbiology. Idu, Abuja, Nigeria, 69-105 (1996).
- Premnathan M. Nakashima H. Kathiresan Rajendran N and Yamamoto N., In vitro anti-human immunodeficiency virus activity of mangrove plants. Indian Journal of Medical Research, 103: 278-281 (1996).
- Roome T. Dar A. Ali S. Naqvi S. and Choudhary I., A study on antioxidant, free radical scavenging, anti-inflammatory and hepatoprotective actions of Aegiceras cor niculatum (stem) extracts, J Ethnopharmacol., 118(3): 514-21 (2008).
- Ross S.A. S.E. Megalla D.W. Bisby and A.H. Aw, Studies for deter mining someantibiotic substance in some Egyptian plants. Screening of some Antimicrobial activity. Fitoterpia, 51: 303-308 (1980).
- WHO. Traditional Medicine Strategy 2002- 2005. WHO Publications. 1-6 (2002).
- Zakaria , Isolation and characterization of active compounds from medicinal plants. Asia Pacific Journal of Pharmacology, 6: 15-20 (1991).

This work is licensed under a Creative Commons Attribution 4.0 International License.





