Manuscript accepted on : August 25, 2009
Published online on: 28-12-2009
Inhibition of Mushroom Tyrosinase by Aliphatic Alcholols
R. Sariri*, F. Aghaghaziani and R. Hassan Sajedi
Department of Biology, Faculty of Science, University of Guilan, Rasht Iran.
Corresponding Author E-mail: sariri@guilan.ac.ir
ABSTRACT: Browning of fruits and vegetables damaged by mechanical injury during harvesting, postharvest storage or processing is one of the main causes of quality loss. Tyrosinase catalyzes the oxidation of phenolic compounds to the corresponding quinines and is responsible for the enzymatic browning of fruits and vegetables. Although the use of compounds such as organic acids, amines and thiols as tyrosinase inhibitors have been studied and reported, but alcohols have received less attention in this regard. This paper reports inhibition of polyphenol oxidase activity by aliphatic alcohols using dopamine hydrochloride as substrate. It was observed that hydroxy containing compounds inhibited tyrosinase activity by a revesible mixed-noncompetitive mechanism. Many kinetic constants, such as Vmax, Kcat and Ki were also obtained in each case.
KEYWORDS: Tyrosinase; inhibition; enzyme kinetics; alcoholic inhibitors
Download this article as:| Copy the following to cite this article: Sariri. R, Aghaghaziani. F, Sajedi. R. H. Inhibition of Mushroom Tyrosinase by Aliphatic Alcholols. Biosci Biotechnol Res Asia 2009;6(2) |
| Copy the following to cite this URL: Sariri. R, Aghaghaziani. F, Sajedi. R. H. Inhibition of Mushroom Tyrosinase by Aliphatic Alcholols. Biosci Biotechnol Res Asia 2009;6(2). Available from: https://www.biotech-asia.org/?p=8639 |
Introduction
Tissue browning in fruits and vegetables damaged by mechanical injury during harvesting, postharvest storage or processing is one of the main causes of quality loss[1, 2]. Enzymatic browning in fruits and vegetables is predominantly catalyzed by a copper-containing enzyme, tyrosinase (EC.1.14.18.1), also called as catecholase or polyphenol oxidase[3-5]. Polyphenol oxidases (PPO) (EC 1.14.18.1) are oxido-reductases that catalyze the hydroxylation of monophenols and the subsequent oxidation of o-diphenols to o-quinones[6-8]. The enzymatic products, o-quinones, are susceptible to oxidation, leading to polymerization and the formation of brown, red or black pigments[5, 6]. The most important endogenous phenolic substrates for PPO in apple and potato sources are chlorogenic acid, catechol, caffeic acid, L-3, 4-dihydroxyphenylalanine (L-DOPA), 4-methylchatechol, p-hydroxyphenylacetic acid (DHPAA), 4-hydroxyphenylpyruvic acid, p-coumaric acid and m– and p-cresol[9]. Polyphenol oxidase mediated browning in raw fruits and vegetables has been known as the major cause of quality deterioration in fruits and vegetables and their derived food products[10]. Enzymatic browning is also catalyzed by another oxidizing enzyme, peroxidase (EC 1.11.1.7). The relative contribution of these two enzymes is still unknown and may differ with plant source[10-14]. Enzymatic browning affects the appearance, organoleptic properties and nutritional quality of food products. The control of enzymatic browning is, therefore, a challenge to the food industry[15-19]. Tyrosinase inhibitors are important in food industry as their activity is of importance in preventing synthesis of melanin in the browning of plants and fruits.
We have previously reported the inhibititory effect of some thiol compounds on tyrosinase[20]. These –SH containing compounds can also act as potent inhibitors on peroxidases[21, 22]. Inhibition of mashroom tyrosinase by many compounds has been studied. It has been stated that the kinetics of inhibition depends highly on the type of substrate[23]. A number of organic compounds including aromatic carboxylic acids[24], sulphites[25], amino acids[26] and aromatic amines[27] have been identified as effective inhibitors of PPO. However, the use of these compounds has been restricted due to their potential hazards. In addition, the use of browning inhibitors in food processing is restricted by considerations relevant to wholesomeness, and effect on taste, flavour, texture, and cost. Browning inhibitors may be classified in accordance with their primary mode of action. In general, various categories of polyphenol oxidase inhibitors are applicable in the prevention of enzymatic browning. These include (1) reducing agents; (2) acidulants; (3) chelating agents; (4) complexing agents; (5) enzyme inhibitors and (6) enzyme treatments [28].
Aliphatic alcohols are expected to act as tyrosinase inhibitors (category 5 of POO inhibitors), although an extended body of research is not present. However, due to their low cost, higher stability, ease of production and little hazard to food, they can be nominated for possible use in food industry to protect fruits and vegetables against enzymatic browning.
In this study, the inhibitory effect on mashroom tyrosiase by some aliphatic alcohols was investigated. The progress of enzymatic reaction was followed using 3-methyl-2-benzothiazolinone hydrazone (MBTH) as the color reagent. The product of tyrosinase activity on dopamine hydrochloride reacted with the amino group in MBTH to produce a deep pink colored complex with a maximum absorption at 503 nm.
Materials and Methods
Chemicals
Mushroom tyrosinase (2033 units/mg) was purchased from Fluka (Zwijndrecht, The Netherlands), Dopamine hydrochloride and MBTH (3-methyl-2-benzothiazolinone hydrazone) from sigma (St. Louis, MO. USA), and all other chemicals from Merck (Darmstadt, Germany) and were reagent grade.
Enzymatic assay
The diphenolase activity of mushroom tyrosinase was measured spectrophotometrically. The enzymatic reaction was initiated by addition of a known amount of the enzyme to a solution of substrate containing dimethyl formamide (DMF and MBTH). DMF was added to the reaction mixture in order to keep the resulting colored complex in solution state during the course of investigations. The progress of the reaction was followed by measuring the intensity of the resulting pink color at 505 nm. A typical reaction mixture with a total volume of 1.0 ml contained, 100 ml enzyme solution, 500 ml substrate solution (60 mM Dopamine hydrochloride mM, 2% (v/v) DMF (dimethyl fomamide), and 5 mM MBTH in 50 mM phosphate buffer), and 400 ml phosphate buffer (pH 6.8). To estimate and compare the activities measured in this study, the molar extinction coefficient of the The MBTH–quinone adducts was used.
Effects of inhibitors on the enzymatic activity
To investigate the effect of alcoholic inhibitors on the activity of enzyme, different concentrations of each inhibitor were pre-incubated with enzymes at room temperature. Tyrosinase activity was then measured by replacing the phosphate buffer with 400 ml of each inhibitor. The 50% inhibition (IC50) of tyrosinase activity was calculated as the concentrations of test samples that inhibited 50% of tyrosinase activity. To obtain IC50 for each inhibitor, VI/V0 was plotted against log [I] for each alcohol.
Kinetic analysis
Enzymatic activities of tyrosinase were investigated at six different substrate concentrations in the presence and absence of IC50 of inhibitors under assay conditions. Michaelis–Menten constant (Km) and maximal velocity (Vmax) of the tyrosinase were determined from the Lineweaver–Burk plots.
Results and Discussions
Many synthetic and natural compounds capable of inhibiting mushroom tyrosinase have been reported so far, most of which are cyclic compound especially phenol types. However, the number of aliphatic compounds acting as tyrosinase inhibitors has been rare to now. Although some reports[29, 30] are found about the inhibitory effect of hydroxyl compounds, but a systematic and classified investigation has not been performed. We, therefore, investigated the inhibitory effect of some aliphatic alcohols on the oxidation of dopamine hydrochloride by tyrosinase. The study was designed so that the result would be able to show the effect of alkyl chain length (methanol, ethanol, 1-propanol, 1-butanol); type of the alcohol (1-propanol and 2-propanol) and number of OH groups (ethylene glycol and glycerol). Figure 1 shows the chemical structures of the alcohols used as inhibitors.
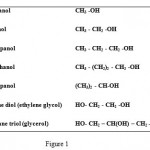 |
Figure 1: The chemical structures of the alcohols used as inhibitor.
|
The rate of tyrosinase reaction on dopamine hydrochloride was studied at room temperature (20ºC) in the absence and presence of inhibitors. A reversible inhibition was obsereved on tyrosinase activity by all alcohols. Typical Michaelis-Menton plots comparing the rate of enzymatic reaction in the presence and absence of alcohols were obtained (not presented here). The hyperbolic dependence of the rate on substrate concentrations confirmed nonlinear regression to the Michaelis equation. The concentrations leading to 50% activity lost (IC50) of all the tested alcohols were calculated using their Dickson plots (Fig. 2) and results are presented in Table I. The values of IC50 in Table I show that the order of potency of primary alcohols is directly related to their alkyl chain length, i.e. 1-buthanol > 1-propanol > ethanol > methanol.
Table I. Kinetic parameters for mushroom tyrosinase in the presence and absence of IC50 of aliphatic alcohols.
| Inhibitor | IC50 (μM) | Km (µM) | Vmax (µmol/min) |
| None | – | 12.4 | 4.8 × 10-3 |
| Methanol | 2400 | 11.7 | 3.5 × 10-3 |
| Ethanol | 2000 | 11.0 | 3.7 × 10-3 |
| 1-Propanol | 1000 | 8.9 | 2.6 × 10-3 |
| 1-Butanol | 800 | 8.2 | 2.7 × 10-3 |
| Ethylene glycol | 2300 | 7.7 | 3.3 × 10-3 |
| 2-Propanol | 2500 | 13.9 | 3.7 × 10-3 |
| Glycerol | 1500 | 10.8 | 3.8 × 10-3 |
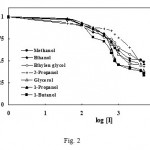 |
Figure 2: Dose-response plots of tyrosinase fractional activity as a function of inhibitor concentration. The value of IC50 is determined as the concentration of inhibitor when V1/V0 = 0.5.
|
In order to show the type of inhibition by each alcohol, the double reciprocal Lineweaver-Burk plots were plotted for all cases. The results obtained from this study showed that the nature of inhibition by aliphatic alcohols was of reversible and either non-competitive or un-competitive type. Figure 3 shows typical Lineweaver-Burk plots obtained in the absence and presence of inhibitors. It is known that a non-competitive inhibitor binds to the enzyme or enzyme substrate complex, and that increasing the substrate concentration cannot reduce the potency of inhibitor. It has been observed that, in addition to its active site, tyrosinase posses an effector site to which the inhibitor can bind [31]. Partial biding of the substrate to the active site may change the conformation of the enzyme so that the hydrophobic pocket (effector site) expands. Therefore, longer alkyl chains would accommodate and bind more tightly, i.e. butanol is the most potent inhibitor among primary alcohols used. Similar results have been reported about the inhibitory effect of alkyl[32] and alkoxy[33] benzoic acids. The kinetic constants (Km and Vmax) were calculated from the double reciprocal plots in the case of each inhibitor (Table I). The value of Km indicates the affinity of an enzyme towards its substrates; the greater the value of Km, the less is the affinity[16, 17]. A similar reasoning applies when 1-propanol is compared to 2-propanol, the long hydrophobic pocket is more suitable for binding of straight alkyl chain in the primary alcohol than the branched compact alkyl chain in the secondary propanol (compare Km values of 8.9 and 13.9 for primary and secondary propanol respectively in Table I). The data in Table I also suggest the number of hydroxyl groups is not as important as the chain length and the alcohol type (consider the IC50 of ethanol/ethylene glycol pair in one hand and 1-propanol/glycerol on the other). There is an important point worth to mention when examining the inhibition of tyrosinase by various alcohols. Primary hydroxyl groups are essential for the potency of alcoholic inhibitors. 1-propanol and glycerol are similar inhibitors due to the presence of primary –OH groups. On the other hand the value of IC50 for 2-propanol is much larger which may be related to the lack of primary hydroxyl group. It can be seen that among the alcoholic inhibitors used in this research, 2-propanol does not change the affinity of tyrosinase towards dopamine hydrochloride, while it reduces the rate (Vmax).
The inhibitory mechanisms of alcohols on mushroom tyrosinase, during the oxidation of dopamine hydrochloride, were determined from Linweaver-Burk double reciprocal plots. Fig. 3 shows the double-reciprocal plots of the enzyme inhibited by alcohols. The results indicated that the plots of 1/V0 versus 1/[S} gave a family of straight lines with different slopes and intersects. Among linear alcohols, methanol and ethanol follow a non-competitive mechanism, while 1-propanol and 1-buthanol behaved un-competitively. This kind of behavior indicates that methanol and ethanol could bind, not only the free enzyme, but also with the enzyme-substrate complex, and their equilibrium constants are the same. On the other hand, 1-propanol and 1-buthanol with longer alkyl chains, can not bind the free enzyme. As binding of the substrate expands the hydrophobic pocket of the inhibitor site, their accommodation into this site would then be possible. The un-altered i.e. smaller hydrophobic pocket is sufficient, however, to accommodate smaller alcoholic tyrosinase inhibitors, methanol and ethanol. The presence of more than one –OH group affects the affinity of the enzyme for its substrate, but the mechanism of inhibition is still of un-competitive type (ethylene glycol and glycerol in Fig. 3). The only secondary alcohol, 2-propanol showed a more complex kinetic, a mixed un-competitive inhibition type with a>1.
Table 2: The value of IC50 for various alcohols used as inhibitors.
| Inhibitor type | IC50 (mM) | Inhibition type | Inhibition
constant (mM) |
| Methanol
Ethanol 1-Propanol 1-Buthanol 2-Propanol Ethylene glycol Glycerol |
2400
2000 1000 800 2500 2300 1500 |
Non-competitive
Non-competitive Un-competitive Un-competitive Mixed Non-competitive Non-competitive |
3.92
4.65 2.53 2.04 3.86 3.28 4.26 |
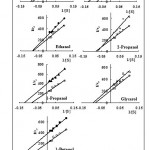 |
Figure 3: Lineweaver-Burk plots, for inhibition of various alcohols, on the oxidation of dopamine hydrochloride by tyrosinase.
|
Fig. 4 shows the Dixon plots for 7 alcoholic inhibitors, the hyperbolic nature of which is characteristic of partial inhibition[34]. A distinguishing feature of a partial inhibitor is that the activity of the enzyme can not be driven to zero even at very high concentrations of the inhibitor. As a true partial inhibition is relatively rare, in the case of more complex alcohols experimental artifacts must be ruled out before concluding that it is acting as a partial inhibitor. In the case of complex chemicals, failure to completely block enzyme activity may be due to their lower solubility.
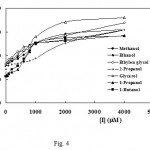 |
Figure 4: Dixon plots, dependence of initial rates of enzymatic reaction on the concentration of inhibitors. The hyperbolic nature of the Dixon plot is a characteristic of partial inhibition.
|
Conclusions
The results obtained in the present study indicate that alcohols are moderate inhibitors of tyrosinase action on dopamine hydrochloride.
Inhibition of tyrosinase by methanol and ethanol follows a mixed type non-competitive kinetics. Higher linear alcohols, 1-propanol and 1-buthanol are un-competitive inhibitors, while the secondary alcohol, 2-propanol, followed a mixed type inhibitory mechanism. Lastly, ethylene glycol and glycerol (polyols) are also un-competitive inhibitors for the action of mushroom tyrosinase upon dopamine hydrochloride. The efficiency of the alcohols as inhibitors depends on the alkyl chain length, branching and, to a lesser degree, the number of hydroxyl groups.
The kinetics of other alcoholic as well as non-alcoholic inhibitors on the activity of mushroom tyrosinase is under further investigations within our group with the aim of construction of a detailed mechanism in each case.
A rare form of inhibition was observed by alcoholic inhibitors used in this research, i.e. partial inhibition. However, to comment this type of inhibition more detailed further investigations are needed.
References
- Sakuraka J., Takahashi S., and Hosoya J., J. Biol. Chem., 261, 9657 (1968).
- Cilento G., Adam W., Photochem. Photobiol., 48, 361 (1988).
- Mayer A. M. Phytochemistry, 26, 11 (1995).
- Whitaker J. R. Polyphenol oxidase, in: D.W.S. Wong (Ed.) Food enzymes: structure and mechanism, Chapman and Hall, New York, 271 (1995).
- Martynez M. V., and Whitaker J. R., Trends in Food Science and Technology, 6(6), 195 (1995).
- Aspuru E. O., and Lourdes Zaton A. M., Spectrochimica Acta, Part A, 55, 2343 (1999).
- Prota. G., Medical Research Reviews, 8, 525 (1988).
- Robbin D. A., and Lontie R. (Ed.)., Copper proteins and copper enzymes Vol. II, Boca Raton, CRC Press, FL, 207 (1984).
- McEvily A. J., Iyengar R., and Otwell W. S., Food Technology, 45, 80 (1991).
- Michael Sullivan L., and Ronald Hatfield D. Crop Sci., 46, 662 (2006).
- Lin Z., Chen L., Zhang W., Process Biochem., 5, 443 (1996).
- Agostini E., Medina M. J., Siliva R., Forchetti M. D. and Tigier H. J., Agric. Food Chem., 45, 596 (1997).
- Everse J. Everse K. E. and Grisham M. B. (Eds.)., Peroxidases in Chemistry and Biology, CRC Press, Boca Raton, FL., 1 (1991).
- Gaspar T., Penel C., and Greppin H. (Eds.)., Plant Peroxidases 1980-1990, Progress and Prospects in Biochemistry and Physiology, 38 (1992).
- Ballantyne A. Stark R., and Selman J. D., Int. J. Food Sci. Technol., 23, 267 (1988).
- Hicks K. B., Sapers G. M., and Seib P. A., U. S. Patent, 4 975, 293 (1990).
- Tong C. B. S., Hicks K. B., Osman S. F., Hotchkiss A. T., and Haine R. M. J. Agri. Food Chem., 43, 592 (1995).
- La Mar G. N., Hernandez G., and Ropp J. S., Biochemistry, 31, 9158 (1992).
- Zaton A. M. L., and Ochao de Aspuru E., FEBS Lett., 734, 192 (1995).
- Sariri R., Mahmoodian J., Khaje Kh., Asian Journal of Chemistry, 18 (1) 8 (2006).
- Sariri R., Sajedi R. H., Jafarian V., and Khaje Kh., Journal of Molecular Liquids, 123, 20 (2006).
- Sariri R., Sajedi R. H., and Jafarian V., Journal of Molecular Liquids, 128, 175 (2006).
- Zollner H., Handbook of Enzyme Inhibitors Part A, Second revised and enlarged Ed., VCH Verlagagesellschaft GmbH, Germany, 367 (1993).
- Chen Q., Song K. K., Qui L., Liu X. D., Huang H., and Guo H. Y., Food Chemistry., 91, 269, (2004).
- Sayavedra-Soto L. A., and Montgomery M. W. J., Food Sci., 51,1531 (1986).
- Kahn V., and Andrawis A., Phytochemistry., 24, 905 (1985).
- Gasowaska B., Kafarski P., and Wojtasek H., Biochimica et Biophysica Actam, 1673, 170 (2004).
- McEvily A. J., Iyengar R., and Otwell W. S., Crit. Rev. Food Sci. Nutr., 32, 253 (1992).
- Nerya O., Musa R., Khatib S., Tamir S., and Vaya J., Phytochemistry, 65 (10), 1389 (2004).
- Jun N., Hong G., and Jun K., Bioorganic & Medicinal Chemistry, 15 (6), 2396 (2007).
- Walker J. R. L., and Wilson E. L., Journal of Science and Food Agriculture, 26, 1825 (1975).
- Huang X. H., Chen Q. X., Wang Q., Song K. K., Wang J., Sha L., and Guan X., Food Chemistry, 94, 1 (2006).
- Chen Q. X., Song K. K., Qiu L., Liu X. D., Huang H., and Guo H. Y., Food Chemistry, 91, 269, (2005).
- Copeland R. A., Enzymes: A Practical introduction to structure, mechanism and data analysis, Second Ed., Wiley VCH, New York, 39, (2006).

This work is licensed under a Creative Commons Attribution 4.0 International License.





