How to Cite | Publication History | PlumX Article Matrix
E. Kibuka-Sebitosi
University of South Africa (UNISA), Centre for African Renaissance Studies, Pretoria South Africa.
Corresponding Author E-mail:: sebitek@unisa.ac.za
ABSTRACT: Female Glossina pallidipes Austen and G. m. morsitans Westwood (Diptera: Glossinidae) were treated with deltamethrin or pyrethrum extract through topical application or by exposure to cloth or glass treated with the same insecticides. Reproductive performance was assessed in terms of survival, pupae number and viability and reproductive deformities and abnormalities within the reproductive systems including abortions. Survival and fecundity of treated flies were significantly reduced (P<0.001). Pupae were small, mostly non-viable, and were arrested at various stages of development. Abortions of egg and all stages of larvae were observed in pregnancy cycles 1-7 following insecticide treatment. Various concentrations ranging from 1-1014 nanograms per micro litre (ng/μL) of insecticide in redistilled acetone topically applied (1μ/fly) on the dorsal thorax of the females, 24 hr after their previous blood meal, resulted in both insecticides in various concentrations causing reproductive abnormalities in both species, including egg re-absorption after ovulation. The sublethal effects were observed to prevent ovulation altogether in some of the flies, which implies effects on the reproductive performances and control strategies for tsetse flies. Sublethal doses could impact negatively on beneficial organisms in the environment where tsetse flies occur mainly riverine, woodlands and forest ecosystems.
KEYWORDS: Glossina; abnormalities; abortions; ovulation; pyrethroids; reproduction; sublethal effects
Download this article as:| Copy the following to cite this article: Sebitosi E. K. Sublethal Effects of Insecticides on the Reproductive Success of the Tsetse Species Glossina Pallidipes and G. M. Morsitans. Biosci Biotechnol Res Asia 2010;7(2) |
| Copy the following to cite this URL: Sebitosi E. K. Sublethal Effects of Insecticides on the Reproductive Success of the Tsetse Species Glossina Pallidipes and G. M. Morsitans. Biosci Biotechnol Res Asia 2010;7(2). Available from: https://www.biotech-asia.org/?p=8850 |
Introduction
Insecticides applied in the field to control tsetse often do not reach their targets in sufficient quantities to kill all of them (Allsopp, 1984), mainly because tsetse resting sites are typically on the underside of leaves and twigs (Moisier, 1912; MacDonald, 1960; Carnevale & Adam, 1971; Challier, 1973; Baldry, 1980), where the flies are less exposed to aerosol droplets. Even when insecticides are applied by helicopters (Baldry et al., 1978; Lee et al., 1978; Molyneaux, et al., 1978; Van Wettere et al., 1978), control measures do not reach 100% effectiveness. Lee et. al., (1980) noted that applications by helicopter result in the deposition of insufficient droplets to kill G. tachinoides Westwood, hence effects on tsetse would largely depend on nocturnal vertical air movements (MacLennan & Cook, 1972). Other authors also questioned the penetration of residual helicopter applications (Johnstone et al., 1974). The use of helicopters to apply dieldrin into dense thickets in Kenya’s Lambwe valley (Le roux & Plat, 1968) revealed similar problems.
Most of the droplets applied during aerial spraying are presumably intercepted by the canopy before reaching tsetse lies. Similarly, after ground spraying of residual insecticides, new flies quickly enter the area (Itard, 1988; Finelle, 1980). Pupae underground are also not directly affected. By the time these adults emerge, most of the insecticides application has been dilute by rain. Flies are exposed to low doses.
Laboratory assessments of toxicity to tsetse are necessary before an insecticide is put into operational use. The term “sublethal effects” has usually describes effects demonstrated in survivors of doses which kill a given proportion of the experimental insects within a defined period of time, for instance 24 hr or 48 hr after exposure. The pioneer work on sublethal effects of insecticides on female tsetse fly G. palpalis is by Baldry (1964). He reported effects on longevity and reproductive performance in the survivors of doses of DDT and dieldrin which killed 72.9% – 97.4% of the flies in the 48hrs after treatment. According to Baldry, insecticides which have deleterious effects on those insects surviving acute poisoning are likely to be more efficient than insecticides with comparable acute toxicity but no such after effects on survivors. Moriaty (1969) reported sublethal effects of insecticides on a wide range of insects, and suggested that, although they must often contribute to the impact of insecticides on pest populations in the field, they have largely been ignored in relation to control.
Kwan and Gatehouse (1978) showed that low doses of insecticides topically applied to G.m.morsitans affected its reproductive activities in several ways (for example, treated flies separated earlier than untreated flies during mating) and reduced the number of pupae surviving. Such doses also affected feeding activities of the tsetse flies. Later on, exposure to endosulfan during the first cycle resulted in F1 mortalities of 39-63%, while the offspring of females treated in the second cycles suffered 19-29% mortality (Kwan and Gatehouse, 1982).
Deltamethrin is particularly effective in killing tsetse (Barlow et al., 1977; Spielberg et al., 1979; Challier et al., 1979; Lavéissiére et al., 1980). It is commonly used to impregnate traps and targets. However, when these traps and targets are left in the field under the tropical sun and rain, the insecticides breakdown rapidly. In Zimbabwe, older techniques employing DDT-impregnated targets also failed to maintain toxicity under the tropical environment conditions (Allsopp, 1984). Hence, under field conditions, tsetse flies are often exposed to sublethal doses of insecticides in typical control; campaigns. Deltamethrin is potentially the most valuable pyrethroid for tsetse control (Hadaway, 1977; Spielberger et al., 1979; Opiyo et al., 1988). It is one of the photostable pyrethroids, its stability having been achieved by modifying the two photo-labile zones of the permethrin structure (Elliot et al., 1978). Preliminary laboratory studies involving topical application techniques demonstrated that it had an intrinsic toxicity to Glossina species approximately 125 times higher than that of dieldrin (Barlow and Hadaway, 1974). A number of workers have demonstrated the potential of deltamethrin for future tsetse control programmes (Barlow et al., 1977; Challier et al., 1977; Hadaway, 1978; Spielberger et al., 1979; Quinlan and Gatehouse, 1981). Trials on ground (Spielberger et al., 1979), in mixtures with endosulfan (Andrews et al., 1983), in traps and targets (Lavéissiére et al., 1981), have revealed the possibility of using extremely low doses for residual and aerosol applications for tsetse control. More field trials and laboratory bioassays are required in order to firmly establish deltamethrin as an effective substitute of the well-established insecticides in tsetse control.
Field and laboratory observations of insecticides on Glossina
Accounts of the use of dieldrin and endosulfan in Glossina control have been given by Spielberger et al. (1977) and Davies (1978). Earlier on, Lee et al., (1967) had assessed the effects and economics of aerial applications of pyrethrum as an aerosol spray against G. pallidipes in Zambia. The early ground-spraying operations were reviewed and successful campaigns have been described by Davies (1971) for Nigeria, while Lavéissiére et al., (1981) observed a reduction in apparent density by trapping after trapping riverine tsetse flies with screens impregnated with insecticide.
Tsetse are adenotrophic viviparous insects, whose pregnancy cycle dynamics are often unknown. The reproductive abnormalities observed in the females are described in detail in this paper in relation to the insecticide impact on the reproductive potential and eventual effects on the population dynamics. The low fecundity of tsetse makes them particularly vulnerable targets for insecticides. Control methods should focus on disrupting physiological processes in insect vectors. Reports on sublethal effects are few but include the observation that low doses may indirectly result in death for example by starvation (Quinlan & Gatehouse, 1981; Baldry, 1964; Sebitosi & Chaudhry, et al., 1988). Despite these studies, sublethal effects of insecticides on the reproduction of G. pallidipes and G.m.morsitans remain unknown. The study presented here investigated the effects of sub-lethal doses of insecticides on the reproduction success of tsetse.
Material and methods
Tsetse
Female mated tsetse flies of two species Glossina pallidipes and Glossina morsitans morsitans were used in these experiments. G. pallidipes adults were collected from Nguruman near Magadi town, 70 Km southwest of Nairobi, Kenya where the species inhabits a wooded bushland and open grass alluvial plains of semi-arid land. The Tsetse flies were collected in biconical traps (Challier and Lavéissiére, 1965) baited with acetone and cow urine. They were maintained in an insectary at 250C; 70-80% relative humidity. Flies were fed daily with rabbit ears and held for a period of one week before treatment o screen out mortalities due to handling and were allowed to acclimatize to laboratory conditions. Pregnant females recognized by the presence of third instar larvae in utero were separated out to synchronize larviposition and the next pregnancy cycles.
Glossina m. morsitans were obtained from the International Centre of insect Physiology and ecology (ICIPE) colony which originated from Langford, Bristol, U.K, in 1965. They were kept under similar conditions like G.pallidipe.
Insecticides
Deltamethrin (98%), pyrethrum extract (25%) from Wellcome Kenya Ltd were dissolved in acetone at doses between -10 and 15ng and applied in a volume of one micro litre to the dorsal thorax of 10-20 flies per replicate, without prior anaesthesia. Control tsetse flies were treated with 1µl acetone. All treated flies were maintained in the insectary kept at 25 0C and 70% R. The flies were observed for abnormalities.
Reproductive abnormalities &Tsetse fly dissections
The females were dissected in a drop of normal saline (0.9 NaCl) after they died. Within 24 hrs of the fly’s death, the female’s legs and wings were removed, and then the fly was put on a glass slide dorsal ventrally. In a drop of normal saline, the second abdominal segment was gently pulled out using fine forceps, while the thorax was firmly held on the slide. The ovaries could be seen as white oval structures. The Reproductive abnormalities were determined according to Saunders (1962) and Challier (1965) and suspected abortions recorded according to Madubunyi (1975). The ovaries of each fly were examined for configuration and evidence of ovulation through the presence of follicular relics and the number of eggs. The normal ages of death were established according to Saunders (1962) ovarian dissections.
Progeny from sublethal doses treated females
The pupae from all the females were maintained singly in plastic tubes until they emerged. A record of incubation periods was kept. The feeding frequency and the actual blood meal size by low dose treated females were also kept and results of feeding patterns and behaviour published else where.
Results
Normal tsetse fly female reproductive system
The female reproductive system is comprised by a uterus and two fallopian tubes on either side of the uterus (Fig. 1). One egg develops to maturity, goes down the tube into the uterus where it develops into a larva. The larvae feeds on the uterine glands until it develops into a third instar stage when it is larviposited. The larvae pupates within 1 hr of larviposition.
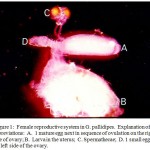 |
Figure 1: Female reproductive system in G. pallidipes. Explanation of abbreviations: A. 1 mature egg next in sequence of ovulation on the right side of ovary; B. Larva in the uterus; C. Spermathecae; D. 1 small egg on the left side of the ovary. |
Reproductive abnormalities
The females treated with pyrethrum extract and deltamethrin showed reproductive abnormalities in their reproductive systems. These included:
Abnormality 1: egg retention: Egg retention in the ovary where by the egg failed to go down into the uterus. The next egg in sequence therefore began to develop thus making 2 mature eggs appear in the ovaries on each side (Fig. 2 &3). These eggs afterwards started to undergo resorption, appearing brown in colour, with patches of yolk (Fig. 4). The abnormality is having 2 mature eggs on either side of the ovary.
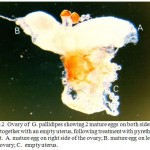 |
Figure 2: Ovary of G. pallidipes showing 2 mature eggs on both sides of the ovary together with an empty uterus, following treatment with pyrethrum extract. A. mature egg on right side of the ovary; B. mature egg on left side of the ovary; C. empty uterus. |
 |
Figure 3: Egg accumulation in the ovaries of G. pallidipes following treatment with pyrethrum extract. A. mature egg; B. Spermathecae.
|
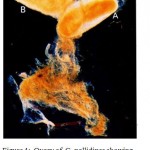 |
Figure 4: Ovary of G. pallidipes showing resorption of eggs (brown in colour) in both the ovaries following treatment with pyrethrum extract. A. egg on right side of the ovary undergoing resorption; B egg on left side of the ovary undergoing resorption.
|
Abnormality 2: larvae retardation: With the abnormality of having 2 mature eggs in the ovary, although these eggs are mature, there were a 1st instar larva in the uterus in some cases. These were abnormalities because, when the egg is mature and ready for ovulation, the larvae in the uterus should be 3rd instar, soon to be larviposited. This indicated retarded development of the larvae. In some cases, there were mature eggs at both sides of the ovary although the uterus was empty. This implied a recent larviposition or an abortion. In a normal situation, the egg on one side of the ovary is mature ( 1.5.mm) while the other side shows the small egg next in sequence, on top of which is usually a very small egg. In the uterus, a 3rd instar larva is normally present, which would be larviposited soon (1-2 days).
The percentage distribution of intra-uterine contents recorded from routine dissections of dead female G. pallidipes and G. m. morsitans treated with deltamethrin given in Table 1 shows high frequencies of abnormalities for both species. The bar graphs in Fig. 5 for G. pallidipes demonstrate an increase in abnormalities with insecticide dose. There was a high percentage of non-ovulation for G. pallidipes (33%), and egg reabsorption (17%) in G. m. morsitnas. However, no egg blocked oviduct or larvae were present in the uterus of G. m. morsitans. In G. pallidipes all types of reproductive abnormalities were observed (Fig. 5). Both tsetse flies revealed relationships of a positive correlation between the percentage of females with reproductive abnormalities and the increasing dose of insecticides. This increase was, however, only up to appoint (3 ng) marking the end of low doses. After that, the trend sharply dropped.
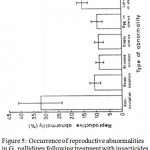 |
Figure 5: Occurrence of reproductive abnormalities in G. pallidipes following treatment with insecticides (data pooled for the two insecticides).
|
Egg development
Normally, the ovary of a fly ready to begin a new pregnancy cycle or to ovulate would have one mature egg next in sequence of ovulation on one side of the ovary (Fig. 1), while the other side has a small egg. Abnormal development in an ovary (reproductive abnormalities, Fig.2 ), included egg retention in one side of the ovary ( 32 %) so that the next egg in sequence began to develop. Two mature eggs then appeared in the ovaries. These eggs soon started undergoing resorption (11 %). They were brown in colour, with patches of yolk reabsorbed. The normal mature eggs was white and compact, with no signs of egg reabsorption as shown.
Other kinds of reproductive abnormalities included two mature eggs, one on each side of the ovary instead of having only on one side, but with an empty uterus (Fig.3). Also cases with two mature eggs one on either side of the ovary but with a 1st or 2nd instar larva were observed. This was an abnormality because when the egg is mature and ready for ovulation, the larva in the uterus should have been a 3rd instar, soon to be larviposited. This observation indicated retarded development of the larva. Some females had up to 6 eggs in the ovaries, which was a most abnormal situation (Fig.4).
Larval development and abortions
Treatment of females with either insecticide resulted in either rapid expulsion of egg within 24 hr of treatment or delayed abortion of larvae (1st, 2nd and 3rd instar,Figure 6).
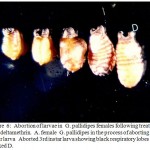 |
Figure 6: Abortion of larvae in G. pallidipes females following treatment with deltamethrin. A. female G. pallidipes in the process of aborting a 3rd instar larva Aborted 3rd instar larva showing black respiratory lobes marked D. |
Generally, higher percentage of non-ovulation egg resorption, and empty uteri were recorded for low doses than the control, however, there were slight differences between the treatments. While there were more flies with non ovulation with the low dose (1 ng), there was more empty uteri and egg resorption with higher doses (5-10ng). Flies appeared to be more affected by the higher doses inducing abortions and expelling eggs (hence the empty uteri). On the other hand, there was an accumulation of eggs in the ovaries with los doses treatment.
The empty uteri implies suspected abortions when the next egg in sequence is mature (Table 1). There was, however, no significant positive correlation between the percentage reproductive abnormality and the insecticide (P<0.05).
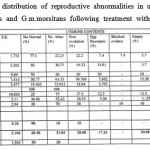 |
Table 1: Percentage distribution of reproductive abnormalities in uterine contents of female G. pallidipes and G.m.morsitans following treatment with pyrethrum extract.
|
On the frequencies of the different reproductive abnormalities, flies with non ovulation were most abundant followed by egg resorption. There was no consistency in the ranked frequencies of the egg, second or third instar larva bearing flies. The mean percentage for the two species of flies obtained for the reproductive abnormalities were as follows: G. pallidipes, normal females= 42.0%, abnormal females= 57.3 %, and for m. morsitans, normal females = 53.61%, abnormal females = 61.7%
Discussion
The dynamics of tsetse fly pregnancy is given by Denlinger, (1978) and the susceptibility of topical application of dieldrin in tsetse in unsprayed and sprayed populations in Kenya (Turner and Golder, 1986) provide a background for the dynamics of tsetse and insecticide interactions. The fact that the percentage of flies with reproductive abnormalities increased with the increasing concentration of insecticide up to a certain point suggest that flies exposed to low doses are affected in their reproductive systems. This implies that flies can tolerate the low dose to a certain extent, but prevent ovulation to occur which subsequently are reabsorbed, where as the higher doses result in ovulation. The most common abnormality was non ovulation. The development of the next egg in sequence of the opposite side of the ovary and the gradual accumulation of the non ovulated eggs mean that the ovaries are stimulated to produce eggs. In other words, low doses of pyrethrum seem to be stimulating egg maturation in tsetse flies. Similar reports have been observed in the spotted lady beetle Henosepilachna vigintioctopuncata (Kono & Ozeki., 1987), who observed that pyrethroids (flucythriante, permethrin and cypermethrin) at 0.05 µl caused about half the females to complete egg maturation, and were also showed to have stimulating effects to vitellogenesis.
This fact further implies that probably low doses caused an increase in the reproductive abnormalities which the inset tolerates. The two species showed similar patterns of abnormalities, which reflect abnormalities due to treatments for both tsetse flies. The higher doses on the other hand, caused egg re-absorption and high numbers of empty uteri which signified abortions.
As to the influence of insecticides of insecticides on the egg maturation without ovulation (non ovulation category) it is logical to speculate that since the pyrethroids used are nerve stimulating, they must have caused a release of neurosecretory substances in the females. These in turn induced a hormone responsible for the vitellogenesis and egg maturation to be released, as may be supported by reports on a hyperglycaemic hormone by DDT in Periplaneta Americana (Grant and Leeling, 1972) and by α-BHC in Schistocerca gregaria (Samaranayaka, 1974) and by bioresmethrin (Sing and Orchard, 1983). These results were in accordance with the action of bioresmethrin (Sing and Orchard, 1983) on the electrical activity of neurosecretory cells of insects.
These results suggest that the stimulating action of deltamethrin and pyrethrum extract at low doses can be manifested in vivo and be used to induce reproductive abnormalities, which in future may be a form of control in tsetse flies. All in all, sublethal doses have an adverse effect on the progeny of the treated females. The question that arises in what proportion of the population was affected. In this study, it has been demonstrated that at least 40% emerged abnormally, but did not survive up to 10 days means that a total of 65% was affected. In the field situation, this is likely to increase thus contributing to mortality after spray application. The implication of these results to the environment and non-target beneficial organisms is fundamental. It implies that insecticides sprayed can interfere with the reproduction of beneficial insects for instance bees and butterflies with detrimental effects to the eco systems and the environment.
Application of pyrethroids to intact insects causes behavioural and physiological manifestations of poisoning superficially resembling those of DDT. Initially, there is a period of sensory hyperexcitation which leads to loss of coordination, ataxia, prostration, convulsions and eventual death. The pyrethroids effects however, are more rapid than DDT. Lowenstein (1942), while working on the nerve cord of cockroach Blattela germanica, and using electrophysiology in vitro, observed an excitory phase induced by pyrethrins, and then a blockage of the nerve conduction mechanism. The author adduced evidence to show that the time interval between the excitory phase and the blockage phase depends on the dose used. The higher the dose, the shorter the time between the two phases. In contrast, low doses tended to increase the duration of the excitory phase, but always allow a return to the normal conduction rate. In vitro investigations have shown that pyrethrins and pyrethroids have a rapid stimulating action on the nervous system of insects. Various possible modes of action on the nervous system in relation to the visible intoxication symptoms show that motor unit discharges originate in peripheral motor nerve endings. Trains of such discharges may well be the cause of convulsions, suffered by the poisoned insects (Roussel, 1982).
Takken et al. (1986), using integrated control of biconical traps and targets impregnated with deltamethrin and the sterile insect technique in Nigeria, managed to reduce the G. palpalis palpalis population by more than 90%, but failed to eradicate it. An unsuccessful attempt to eradicate G. pallidipes from the Lambwe Valley using sequential aerial application of endosulfan was described by Turner and Brightwell (1986). Vale et al. (1988) used targets impregnated with dieldrin and deltamethrin and baited with 1-octen-3-ol attractants, to reduce the populations of G. morsitans morsitans and G. pallidipes in Zimbabwe. Several authors including Torr (1985) and Lavéissiére et al., (1986) described experiments with synthetic pyrethroids with Glossina.
In this study, sublethal doses of insecticides have been shown to affect the reproduction of tsetse flies through abnormalities. This phenomenon is not new to insects. Duncan (1963) had exposed female Aedes aegypti to Dieldrin at LD2 to LD5 24 hr and observed an inhibition of feeding. The minor effects of the solvent and the insecticides on blood meal size and the frequency of feeding when blood was offered daily was recommended for further investigation.
Tsetse show interspecific as well as intraspecific variation in susceptibility to an insecticide. Generally, teneral male and female flies, and older fed males are similar in susceptibility to organochlorine insecticides. Fed pregnant females are appreciably less susceptible by a factor of 4-9 (Burnett, 1962; Irving, 1968; Kwan et al., 1982). Pregnant females are also more tolerant of synthetic pyrethroids (Burnett, 1962; Hadaway, 1972; Kwan et al., 1982; Riordan, 1987; Turner and Golder, 1986).
86). The results have confirmed earlier observation that pregnant females tolerate insecticide treatment through abortions.
The results suggest that the stimulating action of deltamethrin and pyrethrum extract at low doses can be manifested in vivo and be used to induce reproductive abnormalities. Results from the survival have shown that both deltamethrin and pyrethrum extract reduced the survival of G. m. morsitans but not of G. pallidipes. This difference may be genetic in origin. Nevertheless it implies that sublethal doses of these insecticides have an adverse effect on the survival of the flies. If such flies do not survive well, then these low doses do make a significant contribution to the fly mortality after spray application. All in all, sublethal doses have an adverse effect on the progeny of the treated females. The question that arises is at what stage and what proportion of the population could they be effective?
Irregular feeding was the main cause of abortions, an observation similar to that of Mellanby (1937). The same author noted that egg maturation and “milk” production are dependent upon the nutrients made available from the blood meal of the female, and that the pupal weight could be directly connected with the total amount of blood taken during the pregnancy cycle.
Burnett (1963), Irving (1968), Kwan et al. (1982) and Riordan (1987) all reported an increase in tolerance of pregnant flies to insecticide treatment. Burnett (1963) suggested that tolerance raised for a low dose of fenthion was due to diffusion of insecticide into the in utero embryo. Similar diversion of DDT into the in utero larva was termed “inert storage” by Irving (1968), and Kwan et al (1982) suggested that partitioning of endosulfan into lipids in the maternal fat body during early pregnancy and in uterine milk during late pregnancy, explained increasing in tolerance through each pregnancy cycle.
Abortion by tsetse flies living in natural conditions has been recognised for many years and it was suggested by Buxton (1955) that this could be due to undernourishment of individual flies. Estimates of abortion rates in wild populations have varied considerably, ranging from 0.04% (Harley, 1966) to 0-12 % (Okiwelu (1977) for G.brevipalpis in Uganda and G.m.morsitans in Zambia respectively. Using a precise method of detecting abortions, Madubunyi (1975) recorded a rate of 8.72 % of parous flies of a population of G.m.morsitans in Zambia and later he recorded 7% in a large sample of the same population. Denlinger (1975) reported abortion rate of 6.9% for acetone treated flies which were fed daily.
The mechanism and mode of action of tsetse abortifacients are not very well understood. It has been suggested that they can act directly or indirectly. The direct action influences the parturition; indirectly, they may act by turning off the milk gland. Other possible modes of action include the disruption of the in utero larval morphogenesis and the desynhcronisation of the female neuro endocrine integration of normal pregnancy. Denlinger (1975) working on G.m .morsitans observed very rapid abortions 2-24 hr after Juvenile Hormone Analogue treatment. He suggested that an affect on the maternal milk gland was not the cause of abortion, because the resultant starvation of the in utero progeny would not have proceeded sufficiently far in such a short period. The author also reported that injection of the antichlolinesterase substance, physostigmine, caused rapid abortion in G. morsitans . He concluded that compounds acting on the neuromuscular processes could also act as abortifacients.
Langley (1977) recognized that starvation of the pregnant fly or larval death of the embryo in the uterus can result in abortion. There is evidence that brain neurosecretory cells are involved in the control of parturition (Foster, 1974) and that the release of neurosecretions reaches a peak at the time of parturition (Ejezie & Davey, 1974). Langley (1977) suggested that normal parturition is initiated by the maternal neuroendocrine system which causes muscular relaxation of the vaginal sphincter and the fully developed larva escapes from the uterus by its own reverse peristaltic movements.
A significant effect on the larval development was observed for the two insecticides on the two species. The egg and immature larva would have little muscular activity. It is therefore likely that abortion resulted by inducing relaxation of the maternal muscles, opening of the vaginal sphincter and smoothering of the uterine wall.
Irving (1968) reported that the insecticides were channelled to the developing larvae through the milk. Both insecticides induced abortions in the egg stages as well as in all three larval instars. Although the insecticides were applied to individuals in early pregnancy, they affected the abortion of 2nd and 3rd instar larvae. The 3rd instars which were aborted were not fully grown, and they often failed to pupate. This observation tends to agree with Irving’s findings that the developing larvae incorporate the insecticide via the milk hence they are the most frequently aborted, a direct action of the insecticides.
The time between insecticide application and abortion was much shorter with deltamethrin than with the pyrethrum extract. Deltamethrin is recommended for utilization in the tsetse control programmes (Barlow et al., 1977; Lavéissière et al., 1980; Spielberger et al., 1979). It is used in impregnated traps and targets for tsetse control.
It is clear that low-dose insecticide effects on reproduction do cause a significant contribution to the overall mortality of the tsetse fly population. When insecticides are applied therefore, those flies that get sublethal doses, although they are not affected in terms of reducing their feeding behaviour, they are affected through induction of abortions, reproductive abnormalities inside their ovaries, and pupal development retardation.
It has been demonstrated that sublethal doses of pyrethrum extract and deltamethrin affect tsetse reproduction through: abortions of eggs and larvae, retarding pupal development and viability, reducing post-treatment survival, and reproductive abnormalities in the reproductive system. However these studies have not shown the actual mode of action of these insecticides. Further studies are recommended using radio labelled deltamethrin in vivo, which would show where the sites of action are. Also, the effect of these insecticides on the F2 and F3 should be investigated to establish the long term effects on the progeny, if any.
Acknowledgements
I wish to thank Sandra and Benjamin for their moral support as I wrote this paper. I would like to acknowledge ICIPE for the facilities and technical support and insectary. I thank International Development Research Centre (IDRC) for the grant offered to me during this research.
References
- Allsop, R. (1984) Control of tsetse flies using insecticides a review and future prospects. Bulletin of Entomological Research, 74, 1-23.
- Andrews, M., Flower, L.S., Johnstone, D.R. & Turner, C.R. (1983) Spray droplet assessment and insecticide drift studies during the large scale aerial application of endosulfan to control Glossina morsitans in Botswana. Tropical Pest Management, 29, 239-248.
- Baldry, D.A. T. (1964) Some effects of sublethal quantities of insecticides on the longevity and fecundity of females of Glossina palpalis (R-D). World Health Organization (WHO) Vector control, 90.
- Baldry, D.A.T. (1980) Local distribution and ecology of Glossina palpalis and G. tachinoides in forest foci of West African human trypanosomiasis, with special reference to associations between peri-domestic tsetse and their hosts. Insect Science and its Application, 1, 85-93.
- Baldry, D.A.T., Molyneux, D.H. & Van Wettere, P. (1978) The experimental application of insecticides from a helicopter for the control of riverine populations of Glossina tachinoides in West Africa. V. Evaluation of decamethrin applied as a spray. Pesticide Articles and News Summary, 24, 447-454.
- Barlow F. & Hadaway, A. B. (1974) Some aspects of the use of solvents in ULV formations. British Crop Protection Council Monograph, 11, 84-93.
- Barlow, F., Hadaway, A.B., Flower, L.S., Grose, J.E., & Turner, C.R. (1977). Some laboratory investigations relevant to the possible use of new pyrethroids in Control of mosquitoes and tsetse flies. Pesticide Science, 8, 291-300.
- Burnett, G.F. (1962) The susceptibility of tsetse flies to topical application of insecticides. III. The effects of age and pregnancy on the susceptibility of adults of Glossina morsitans Westwood. Bulletin entomological Research, 53, 337-345.
- Carnevale, P. & Adam, J.P. (1971) Contributions to the biological study ofGlossina palpalis palpalis R-D in the popular Republic of Congo. pp.207-211 in International Scientific Council for Trypanosomiasis Research (ISCT) Thirtheen Meeting, Lagos, Nigeria.328 pp. Niamey, Niger. Organization of African Unity (OAU) Publications Bureau, Maison de l’Afrique.
- Challier, A. (1965) Amélioration de la méthode de détermination de l’âge Physiologique des glossines. Bulletin de Société Pathologie exotique, 58, 250-259.
- Challier, A. (1973) Ecologie de Glossina palpalis gambiensis Vanderplank, 1949 (Diptera: Muscidae) en Savane d’Afrique occidentale 274 pp. Paris France ORSTOM (Memoires ORSTOM no.64).
- Challier, A., Eyrand, M. & Sales, S. (1977) Effectiveness on tsetse flies of residual deposits of three pyrethroids OMS-1821, 1998 and 2002, in Comparison with an organochloride OMS 570, in a Sudanian savanna galley forest, Upper Volta. WHO. Unpublished document. World Health Organization (WHO)/VBC, 77/ 670.
- Davies, J.E. (1981) Insecticide drift and reinvasion of spray blocks in aerial spraying experiments against G. morsitans centralis Machado. Bulletin of Entomological Research, 71, 499-508.
- Denlinger, D.L. & Ma, W.C. (1974) Dynamics of the pregnancy cycle in the tsetse morsitans. Journal of Insect Physiology, 20, 1015-1016.
- uncan, J. (1963) Post-treatment effects of sublethal doses of dieldrin on the mosquito Aedes aegypti L. Annals of applied Biology, 52, 1-6.
- Elliot, M., Janes, N.F. & Potter, C. (1978) The future of pyrethroids in insect control. Annual Review of Entomology, 23, 443-690.
- Hadaway, A.B. (1972) Toxicity of insecticides to tsetse flies. Bulletin of the World Health Organization, 46, 353-362.
- Hadaway, A.B. (1977) The role of laboratory testing of insecticides for tsetse fly Transactions of the royal Society Tropical Medicine and Hygiene, 71, 6-7.
- Hadaway, A.B. (1978) Post-treatment temperature and the toxicity of some insecticides to tsetse flies. World Health Organization (WHO)/VBC, 78/ 693.
- Irving, N.S. (1968) The absorption and storage of insecticide by the utero larva of the tsetse fly Glossina pallidipes Aust. Bulletin of Entomological Research, 58, 221-226.
- Itard, J. (1966) Cycle de l’oogenese chez les femelles de l’age physiologique. Revelation Elevation Medicine Veterinaries Pays Tropical 19, 331.
- Johnstone, D.R., Huntingdon, K.A. and Coutts, H.H. (1974) Penetration of spray droplets applied by helicopter into a riverine forest habitat of tsetse flies in West Africa. Agricultural Aviation, 16 (3), 71-82.
- Kwan, W.H. & Gatehouse, A.G. (1978) The effects of low doses of three insecticides on activity, feeding, reproductive performance and survival in Glossina morsitans morsitans (Glossinidae). Entomologia experimentalis et applicata, 23, 201-221.
- Kwan, W.H., Gatehouse, A.G. & Kerridge, E. (1982) Effects of endosulfan on pregnant females of Glossina morsitans morsitans Westwood (Diptera: Glossinidae) and their offspring. Bulletin of Entomological Research, 72, 391-401.
- Lavéissière, C., Couret, D.H. and Eouzan, J.P. (1986) La campagne Cote de lutte contre la trypanosomiase humaine dans le foyer de vavoua (Cote d’Ivoire). 3. Resultats des evaluations entomologiques. Cah. ORSTOM, Ser. Entomol. Med. Parasit, 24, 7-20.
- Lee, C. W., Park, P.O. and Gledhill, J.A. (1967) A trial to control tsetse flies by aerial application of aerosols in Borotseland, Zambia. Miscellaneous Report of Tropical Pesticide Research Institute, 635, 1030.
- Lee, C. W. Parker, J.D. Kultzre, H, Baldry, D.A.T. Beettany, B.W. and Tunstal, J. (1980) The experimental application of insecticides from a helicopter for the control of riverine populations of Glossina tachnoides in West Africa. VII. Studies on the physical properties of sprays of endosulfan and deltamethrin applied to G. tachnoides habitats in the R. Komoe Valley, Upper Volta. Tropical Pest Management, 26, 377-384.
- Leroux, J.G. and Platt, D. C. (1968) Application of dieldrin invert emulsion by helicopter for tsetse control. pp219-229. In International Scientific Committee for Trypanosomiasis Research Twelfth Meeting. Bangui.
- Maclennan, K.J.R. and Cook, M.G. (1972) The resting behaviour of Glossina morsitans Newst, in the northern Guinea vegetation Zone in relation to control using insecticides. Entomologist, 105, 144-152.
- Madubunyi, L.C. (1975) A technique for detecting abortions in wild populations of Glossina species. pp 477-485: In Sterility principle for insect control 1974. Vienna International Atomic Energy Agency (Publ. No.IAEA-SM-186/32).
- Moisier, B. (1912) Notes on the haunts and habitats of Glossina tachnoides near Geidam, Bornu province, Northern Nigeria. Bulletin of entomological Research, 3, 195-202.
- Molyneux, D.H, Baldry, D.A.T., Van Wettere, P., Takken, W. & de Raadt, P. (1978) The experimental application of insecticides from a helicopter for the control of riverine populations of Glossina tachnoides in West Africa. 1. Objectives, experimental area and insecticides evaluated. Pesticide Articles and News Summary , 24, 391-403.
- Moriarty, F. (1969) The sublethal effects of synthetic insecticides on insects. Biological Reviews, 44, 321-357.
- Opiyo, E.A., Dolan R.B., Njogu R. Sayer, P.D. and Mgutu, S.P. (1988) Tsetse control on Galan ranch. In: OAU/STRC (13, (6053) 434-437.
- Quinlan, R.T. and Gatehouse, A.G. (1981). Characteristics and implications of knock down of the tsetse fly Glossina morsitans morsitans Westwood. by deltamethrin. Pesticide Science, 12, 439-442.
- Riordan, E.K. (1987). Insecticide tolerance of pregnant females of Glossina palpalis palpalis (RD) (Diptera: Glossinidae).Bulletin of Entomological Research, 77, 22, 213-226.
- Roussel-Uclaff, (1982) Deltamethrin monograph. Roussel-UCLF. London Sebitosi, E.K and M.F.B.Chaudhury, (1988). The influence of sublethal doses of insecticides on the reproduction of Glossina pallidipes and G. morsitans. In proceedings of the Tsetse and Trypanosomiasis control conference, In: OAU/STRC (13, 6053) Mombasa, Kenya.
- Spielberger, V. Naisa, B. K. and Abdurrahim,. U .(1977) Tsetse (Diptera; Glossinidae) eradication by aerial (helicopter ) spraying of persistent insecticides in Nigeria. Bulletin of Entomological Research, 67, 589-598.
- Spielberger, V., Naisa, B.K., Koch, K., Manno, A., Skidmore, P.R. & Coutts, H.H. (1979) Field trials with the synthetic pyrethroids permethrin, cypermethrin and decamethrin against Glossina (Diptera: Glossinidae) in Nigeria. Bulletin of Entomological Research, 69, 667-689.
- Takken, W., Oladunmade, M. A., Dengwat, L., Feldmann, U., Onah, J.A., Tenabe, S.O. & Haman, H. J. (1986). The eradication of Glossina palpalis (Robineau-Desvoidy) (Diptera: Glossinidae) using traps, insecticide-impregnated targets and the sterile insect technique in central Nigeria. Bulletin of Entomological Research, 76, 275-286.
- Torr, J. (1985) The susceptibility of Glossina pallidipes Austen (Diptera: Glossinidae) to insecticide deposits on targets. Bulletin of Entomological Research , 75, 451-485.
- Turner, D.A. & Brightwell, R. 1986. An evaluation of sequencial aerial spraying operation against Glossina pallidipes (Austen (Diptera : Glossinidae) in the Lambwe valley of Kenya: aspects of post-spray recovery and evidence of natural population regulation. Bulletin of Entomological Research, 76, 331-349.
- Turner , D.A. & Golder, T.K. (1986). Susceptibility to topical applications of endosulfan and dieldrin in sprayed and unsprayed populations of Glossina pallidipes Austen (Diptera: Glossinidae) in Kenya. Bulletin of Entomological Research, 76, 519-527.
- Vale, G.A., Hall, D.R. & Gough, A.J.E. (1988) The Olfactory responses of tsetse flies, Glossina spp. (Diptera: Glossinidae), to phenols and urine in the field. Bulletin of Entomological Research,. 78 (2), 293-300.
- Van Wettwere, P., Baldry, D.A.T., Molyneux, D.H., Clarke, J.H., Lee, C.W. & Parker, J.D. (1978) The experimental application of insecticides from a helicopter for the control of riverine populations of Glossina tachnoides in West Africa. IV. Evaluation of insecticides applied as aerosols. Pesticide Articles and News Summary, 24, 435-446.

This work is licensed under a Creative Commons Attribution 4.0 International License.





