How to Cite | Publication History | PlumX Article Matrix
Potential Interactions between Cinnamon and Metformin Treatment in Diabetic Rats
Lujain A. Ashoor and Safaa Y. Qusti*
Department of Biochemistry, Faculty of Science, King Abdulaziz University, Jeddah - 21551 Saudi Arabia.
ABSTRACT: Cinnamon has a beneficial role in improving the symptoms of type 2 diabetes. Concurrent use of cinnamon and the antidiabetic drug “metformin” could have undesirable effects on glucose metabolism. Therefore, the present study investigated the proposed undesirable effect of cinnamon through the interaction between two doses of cinnamon extract and metformin through studying the biochemical parameters related to diabetic syndrome in rats. Ninety male Wistar rats were divided into nine groups. Cinnamon extracts were administered in doses of zero, 300 (low), 600 (high) mg/kg/day to three groups of each, normal, diabetic and metformin-diabetic rats. In normal animals, the high dose of cinnamon extract was able to decrease serum glucose level by 13%, while the two doses of cinnamon did not effect the insulin level. The greatest level of hypoglycemia and the lowest level of serum insulin were obtained with metformin-diabetic rats which received the high dose of cinnamon. Moreover, it is likely that cinnamon extract had similar effects as metformin in lowering the serum lipids of diabetic animals. The combination of metformin with cinnamon extract increased the serum level of HDL-Cholesterol significantly, more than metformin alone and the two doses of cinnamon decreased serum total cholesterol and LDL-C levels. In diabetic rats, the two doses of cinnamon had almost similar reducing effects in ALT, AST and LDH as metformin while the greatest activities were observed with diabetic and metformin-diabetic rats which received high dose of cinnamon. Cinnamon interaction with metformin in diabetic rats might induce DNA damage in hepatocytes, with greater damage being detected at high dose of cinnamon than the low dose. This result could be due to increase in oxidative stress together with poor glycemic control. In conclusion, this study would support the beneficial effect of cinnamon in diabetic rats. While in metformin-diabetic rats, cinnamon would have a synergistic activity compared to the metformin alone which leads to adverse effects on glucose metabolism.
KEYWORDS: Cinnamon; metformin; interactions; type 2 diabetes
Download this article as:| Copy the following to cite this article: Ashoor L. A, Qusti S. Y. Potential Interactions between Cinnamon and Metformin Treatment in Diabetic Rats. Biosci Biotechnol Res Asia 2010;7(2) |
| Copy the following to cite this URL: Ashoor L. A, Qusti S. Y. Potential Interactions between Cinnamon and Metformin Treatment in Diabetic Rats. Biosci Biotechnol Res Asia 2010;7(2). Available from: https://www.biotech-asia.org/?p=8968 |
Introduction
Herbs are used as medicines and nutrition all around the globe. The first written records describing treatment of disease with herbal remedies date back to about 1500 B.C. and are inscribed by the ancient Egyptians on papyrus (Palmer 2004)
Diabetes mellitus is a common and very prevalent disease. Type 2 diabetes is the most common metabolic disease worldwide, with a prevalence estimated to rise from 171 million in 2000 to 355 million in 2030.
The interaction may increase or decrease the effectiveness and/or the side effects of the drugs. It may also result in a new side effect, that is, a side effect not seen with the use of any one drug alone (Ernst 2000). Many traditional folk medicinal herb extracts have been used for the treatment of diabetes mellitus. However, cinnamon is one of these herbs used in Korea, China and Russia for diabetes mellitus.
Over the past two decades, in vitro and in vivo data have been accumulating to support the role of cinnamon on glycemic control (Ziegenfuss et al. 2006).
However, in vitro studies revealed that the cinnamon extract mimics the effect of insulin, which potentiates insulin action in isolated adipocytes (Jarvill-Taylor, Anderson, and Graves 2001) by enhancing insulin-stimulated tyrosine phosphorylation of insulin receptor-β, insulin receptor substrate-1, and phosphatidylinositol 3-kinase in a dose-dependent fashion (Qin et al. 2004).
Herbal medicines, however natural, can cause significant toxic effects, drug interaction and even morbidity or mortality (Zlotogorski and Littner 2004). Based on current evidence from in vitro, in vivo and clinical studies, herbal and other dietary supplements interact with many drugs (Chavez, Jordan, and Chavez 2006). Moreover, if an interaction between a herb and a drug does occur, conventional drugs are usually the culprits because they are more pharmacologically active (Kuhn and Winston 2001).
The likelihood of herb-drug interactions could be higher than drug-drug interactions, if only because drugs usually contain single chemical entities, while almost all herbal medicine products (HMPs) (even single-herb products) contain mixtures of pharmacologically active constituents (Fugh-Berman and Ernst 2001). Herbal drug interactions can be characterized as either pharmacokinetic (PK) or pharmacodynamic (PD) in nature (Mahan and Escott-Stump 2004). The most common used of antidiabetic agents is metformin. It was derived from active guanidine ingredients of French lilac (Galeaofficinalis) (Theorell et al. 1999).
Despite the positive evidence from studies on the beneficial effect of cinnamon extract on diabetes, these plants may trigger unexpected reactions when taken with a combination of oral hypoglycemic agents (metformin). On the other hand, it is true that medicinal herbs are similar to therapeutic drugs in that overdoses can cause problems. Therefore, large doses of cinnamon could theoretically increase or decrease the effect of metformin used to treat diabetes.
The present study was designed to investigate the effects of two doses of cinnamon extract in combination with the antidibetic drug-metformin through studying the biochemical parameters related to diabetic syndrome and the wide distribution of the frequency of the damaged levels resulting from cytotoxicity in host response to herb-drug interaction and whether this interaction can cause DNA damage in the hepatocytes of diabetic rats.
Materials and Methods
Chemicals
Metformin (glucophage 500 mg) was purchased from the local pharmacy in Jeddah. Streptozotocin (STZ), nicotinamide, Dimethyl sulfoxide (DMSO), low melting agarose (LMA), ethidium bromide and ethylene diamine tetra-acetic acid (EDTA) solution were purchased from Sigma (St. Louis, MO, USA). Other chemicals were of analytical grade and were purchased from Sigma Chemicals.
Animals
Healthy male Wister rats aged 8-9 weeks were used, with a mean body weight of 220 ± 20 g, supplied by King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia. Rats were housed in polypropylene cages, maintained under standard conditions (12 h light/12 h dark cycle; 24 ± 1°C; 60-70% humidity), and were fed with a standard rat pellet diet (Grain Silos and Flour Mills organization, Jeddah, Saudi Arabia) and water ad-libitum.Ceylon cinnamon was obtained (purchased) from the local market in Jeddah.
Induction of diabetes
An rat model of type 2 diabetes mellitus was induced (Masielloet al. 1998) in overnight-fasted animals by a single intraperitoneal injection of 60 mg kg−1 streptozotocin 15 min after the intraperitoneal administration of 120 mg kg−1 nicotinamide. Hyperglycemia was confirmed by elevated blood glucose levels determined at 72 h and then on day 7 after injection (Shirwaikar, Rajendran, and Kumar 2004).Only rats with fasting blood glucose higher than 135 mg/dl were used for the experiment (Harkness and Wagner 1989).
Metformin treatment
The dose of metformin was started on day 1 as 50mg/kg and it was increased each day by 50mg/kg until it reaches 250mg/kg on day 5 which is a maintenance dose and then the experimental period of 30 days was started (Marta, Maryse, and Nathalie 2000; Solskovet al. 2008).
Cinnamon extraction
Cinnamon bark was dried and finely powdered in a mechanical mixer. Ten g of finely-powdered cinnamon was weighed and mixed with 100 ml of water and kept in a water path at 60 °C for two hours and filtered using a sterilized filter 0.22 μm pore size (Kreydiyyeh, Usta, and Copti 2000). This extract was diluted with water (1:10) (Kannappan et al. 2006) and administered by gavage to rats.
Experimental design
Rats were randomly divided into nine groups of ten rats each (30 normal; 60 diabetic rats). Group 1 – normal rats (N): considered as control group; Group 2 and 3 – normal rats received cinnamon extract 300 (Lcin) (Qin et al. 2003) and 600 (Hcin) mg/kg/day, respectively, orally by gavage; Group 4 – diabetic control rats (DC): considered as diabetic control group; Group 5 – metformin-diabetic rats (DM): diabetic rats received metformin 250 mg/kg/day orally by gavage (Solskov et al. 2008); Group 6 and 7 – diabetic rats received cinnamon extract 300 (DLcin) and 600 (DHcin) mg/kg/day, respectively, orally by gavage; Group 8 and 9 – diabetic rats treated with both metformin, and cinnamon extract 300 (DMLcin) and 600 (DMHcin) mg/kg/day, respectively, orally by gavage. DNA damage was evaluated at the end of the experiment on hepatocytes.
Blood sample and liver collection
After 30 days of treatment, blood samples were collected under light ether anesthesia retro-orbitally from the inner canthus of the eye using capillary tubes (Micro Hematocrit Capillaries, Mucaps). Blood was collected in fresh vials containing anticoagulant were stored at +4 °C for glucose-6-posphate dehydrogenase analysis, and serum was obtained immediately by centrifugation and frozen at −80 °C until used for the biochemical analysis
After decapitation of the rats, livers were rapidly removed, washed with ice-cold saline and weighed for comet test.
Biochemical assays
Serum glucose was estimated using UV-methods (Kunst, Draeger, and Ziegenhorn 1983), using the kit supplied by Dade Behring Limited, UK. Serum insulin was measured by commercially immunoassay kits from ALPCO Diagnostic, UK. Serum total cholesterol (Stadtman 1957), high density lipoprotein-cholesterol (Castelli et al. 1977) , low density lipoprotein-cholesterol (Gotto 1988),aspartate aminotransferase (Saris 1978), alanine aminotransferase (Wroblewski and Ladue 1956), lactate dehydrogenase (Wacker et al., 1956). Blood glucose-6-phosphate dehydrogenase was estimated by using standard kit (Trinity Biotech, Ireland), which is a modification of the spectrophotometric methods of Kornberg and Horecker(1955) and of Lohr and Waller (1974).
Single Cell Gel Electrophoresis Assay or (Comet Test)
The comet assay is a rapid method for measuring DNA single strand breaks (Singh and Stephen 1997). Analysis and measurement of the comet image parameters were done by using the newly acquired Extended Dynamic Range Imageing (EDRI)software (Elassouliet al., 2007).
Statistical analysis
Analysis of the data was performed by one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test. All data expressed as mean±SD. Differences were considered significant at p <0.05.
Results
In normal rats, the high dose of cinnamon extract was able to decrease the serum glucose by 13%, while the two doses of cinnamon did not effect the insulin level. Regarding to the serum enzymes in normal animals, the only significant effect was detected by the increasing of LDH activity in both two doses of cinnamon compared to normal group ( Table 1).
In diabetic animals, the treatment with metformin resulted decreasing the serum levels of glucose and insulin, the reduction of serum glucose levels of the rats which received either the low or high doses of cinnamon extract were similar to the level of the metformin group, there were no difference in serum insulin between the group received metformin and high dose of cinnamon. On the other hand, the lowest level of serum glucose was obtained with metformin-diabetic rats which received the high dose of cinnamon extract. Furthermore, a non-significant reduction in serum insulin levels were obtained with metformin-diabetic rats receiving the low and high doses of cinnamon extract, respectively, compared to the metformin-diabetic group (Table 2). However, either in diabetic or in metformin-diabetic rats, the two doses of cinnamon decreased serum total cholesterol and LDL-C levels similar to the metformin group. Regarding to the serum HDL-cholesterol in metformin-diabetic rats, present study observed that the two doses of cinnamon extract had the highest values of serum HDL-cholesterol, while they did not differ significantly with the diabetic rats which received the high dose of cinnamon extract group (Table 3). Diabetic groups of metformin, low and high doses of cinnamon increased the G6PDH to achieve the normal level. Moreover, G6PDH were increased in diabetic group which received metformin and cinnamon (low or high). The serum activities of AST, ALT and LDH, in metformin-diabetic rats received the high dose of cinnamon were similar to the diabetic control group and they were higher than the other groups (Table 4). Under fluorescence microscopy, at the end of the experimental period, significant DNA damage was observed in the hepatocytes from metformin-diabetic rats that received the high dose of cinnamon,(Figure 1 and 2), with less DNA damage detected with metfomin-diabetic rats that received the low dose of cinnamon compared to the normal rats (Figure 3).
Discussion
The results of the present study, indicated that when normal animals were administrated with a high dose of cinnamon extract, there was a significant decrease in the level of serum glucose by 13%. These results could be due to that, cinnamon extract would improve insulin action via increasing glucose uptake in normal Wistar rats (Qin et al. 2003). ), and the low dose of cinnamon extract was equal to 3.5 g/day in humans. Moreover, in pre-diabetic subjects, the cinnamon extract had significant decreases in fasting blood glucose by 8.4% compared to subjects in the placebo group (Ziegenfusset al. 2006).
In diabetic rats, the cinnamon (low and high) doses were able to decrease serum glucose level significantly compared to the diabetic control. Consist with this result, Khan et al. (2003) reported a reduction of 18-29% of fasting serum glucose in the three cinnamon groups of 1, 3, or 6 g of cinnamon powder in diabetes. The greater reduction of serum glucose level noted in results of the present study 26-43% compared with Khan et al. (2003), could be due to that they used cinnamon powder instead of aqueous cinnamon.
The great reduction of serum glucose level in metformin-diabetic animals received the high dose of cinnamon caused a hypoglycemic effect that was attributed to the high percentage of mortality of this group (40%), accordance to the adjudicated analysis of causes of death with type 2 diabetic patients (Gerstein et al. 2008). In spite of there were no significant different in serum insulin level between interaction groups and metformin group, This serum insulin reduction could be due to the physiological defense against declining glucose level (Cryer 2001).
The possible reason of these results may be due to the constituents of cinnamon that could had a synergistic activity in relation to the metformin. Consistent explanation with results of the present study, Verspohl, Bauer and Neddermann, (2005) found that, cinnamon acted as a synergetic agonist with insulin in vivo to decrease blood glucose levels after a glucose tolerance test. Moreover, Johansen (1999) reported that, the efficacy of glycemic controlachieved with metformin in reducing hyperglycemia in type 2diabetes mellitus is similar to that of insulin. Same trend was observed by Jarvill-Taylor, Anderson and Graves, (2001) that when cinnamon and insulin were combined in vitro, the effect was greater than was expected, implying a synergistic relationship.
In agreement with the explanation, it has been demonstrated that water-soluble polymeric compounds, methylhydroxychalcon polymers (MHCP), isolated from cinnamon have insulin-enhancing biological activity in the in vitro assay measuring the insulin dependent effects on glucose metabolism and also function as antioxidants effects, which may provide synergistic benefits for the treatment of diabetes (Anderson et al. 2004). Other studies have reported that MHCP in cinnamon was found to be an effective mimetic of insulin (Jarvill-Taylor, Anderson, and Graves 2001). In contrast to results of the present study, Suppapitiporn, Kanpaksi, and Suppapitiporn (2006) found that the cinnamon cassia powder 1.5 g/d did not have any significant difference in reducing fasting plasma glucose in type 2 diabetes patients who they were in combination with their current treatment (metformin). The reasons for these negative results could be due to the small dose of cinnamon and also they used cinnamon in the form of powder.
The reduction in the activity of G6PDH that was obtained in the diabetic control group compared to the normal one was restored when the animal was administrated with metformin, low and high doses of cinnamon extract. These results were in line with Gupta, Nehal and Baquer (1997) who used insulin administration to restore the activity of G6PDH in diabetic rats. In results of the present study the highest significant values of G6PDH were observed with metformin-diabetic rats receiving either the low or high doses of cinnamon extract, therefore, the increase in G6PDH enzyme indicated that cells were under oxidative stress(Ercalet al. 2002). However, either in diabetic or in metformin-diabetic rats, the two doses of cinnamon decreased serum total cholesterol levels similar to the metformin group. In diabetic animals which received the high dose of cinnamon or received metformin with cinnamon (low or high) had the same increasing effect on HDL-cholesterol compared to the metformin group.
These results were in accordance with finding of a previous study by Kim, Hyun and Choung (2006), who reported that, there was a significant decrease in the total cholesterol level whereas increases in HDL–cholesterol levels in cinnamon-treated mice. Moreover, Khan et al. (2003), found that oral cinnamon extract reduced total cholesterol in patient with type 2 diabetes mellitus.
In diabetic rats in this study, cinnamon extract alone or in combination with metformin were able to decrease serum LDL-cholesterol level as metformin. These results were in line with Al Jamal (2009) who reported that the LDL-cholesterol was reduced by 30.3% when type1 diabetic individuals used the 6 g/day of cinnamon for 4 weeks. In addition, a combination of metformin with cinnamon extract was able to increase the serum levels of HDL-Cholesterol, more than metformin alone. Therefore, in general it could be concluded that cinnamon extract has a direct role in lipid metabolism and this effect might improve lipid levels in diabetic subjects as metformin.
The serum activities of AST, ALT and LDH, in metformin-diabetic rats received the high dose of cinnamon were similar to the diabetic control group and they were higher than the other groups. These could be due to the leakage of these enzymes from the liver cytosol into the blood stream (Navarro et al. 1993). Results of the present study showed a relative increase in the activity of LDH, indicated the extent of cell death (Zeevalk and Nicklas 2000). Thus, this study concluded that during severe energy deprivation following hypoglycemia obtained by metformin-diabetic rats receiving high doses of cinnamon, mitochondrial swelling occurs due to mitochondrial permeability transition, which upon recovery can activate processes leading to DNA fragmentation and cell death (Ferrand-Drake, Fribery, and Wieloch 1999).
Cao, Polansky, and Anderson, (2007) tested the safety of the cinnamon extracts on cell viability and DNA damage measured using the comet assay and did not detect any signs of toxicity.Moreover, previous in vitro and in vivo studies have demonstrated that metformin causes an improvement in antioxidant activities in various tissues and acts to limit lipid peroxidation (Srividhya and Anuradha 2002). The results of the present study indicated that cinnamon interaction with metformin in diabetic rats was able to induce DNA damage in hepatocytes, with greater damage being detected at high dose of cinnamon than the low dose.
The effect of high dose cinnamon significantly increased the DNA damage in the liver cells. Therefore, it could be suggested that metformin failed to protect against DNA damage and lipid peroxidation in metformin-diabetic animal which received a high dose of cinnamon. In agreement with our results, Onaranet al. (2006) indicated that pharmacological concentrations of metformin are unable to protect against DNA damage induced by a pro-oxidant stimulus in cultured human lymphocytes, despite its antioxidant properties. In contrast, results of Aleisaet al. (2008) demonstrate that, the treatment with metformin might be useful to protect hepatic toxicity. The exact mechanism of action is not known.
Therefore, the increase in oxidative stress together with poor glycemic control could enhance the damage of the biological macromolecules such as protein, lipid and DNA (Blasiaket al. 2004). According to cytogenetic impact, it would be interesting to explore the chemical interaction of cinnamon with metformin with individuals regarding the degree of apoptotic changes, cellular vaculation and nuclear lysis on the dose and time exposure after 24 hours, one week, two weeks and three weeks is needed.
 |
Figure 1: Digital image of the hepatocytes from metformin-diabetic rats.
|
Table 1.Effects of the low and high doses of cinnamon extract on serum parameters in normal rats*.
| serum parameters | N | Lcin | Hcin | Significance |
| Glu [mg/dl] | 71.8a ± 7.0 | 72.4a ± 9.4 | 62.8b ± 9.4 | S |
| Insulin [ng/l] | 165a ± 34 | 179a ± 60 | 187a ± 39 | NS |
| TC [mmol/L] | 1.39a ± 0.21 | 1.57b ± 0.14 | 1.46a,b ± 0.20 | S |
| HDL-C [mmol/L] | 0.402a ± 0.007 | 0.404a ± 0.026 | 0.429a ± 0.036 | NS |
| LDL-C [mmol/L] | 0.840a ± 0.165 | 0.975a ± 0.071 | 0.950a ± 0.160 | NS |
| **G6PDH [U/g Hb] | 11.8a ± 1.1 | 12.3a ± 0.6 | 12.2a ± 1.8 | NS |
| AST [U/l] | 107a ± 16 | 112a ± 9 | 118a ± 11 | NS |
| ALT [U/l] | 47.1a ± 4.7 | 43.8a ± 4.0 | 47.3a ± 4.7 | NS |
| LDH [U/l] | 809a ± 63 | 1051b ± 162 | 1007b ± 139 | S |
Values are presented as means with their standard deviations. Values not sharing a common superscript letter differ significantly at p>05 (one-way ANOVA and LSD-tests), S= Significant (p> 0.05) and NS= Non Significant (p> 0.05).
Glu: glucose, TC: total cholesterol, HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, G6PDH: glucose-6-phsphate dehydrogenase.
N: Normal rats; Lcin: Normal rats received low dose of cinnamon (300 mg/kg/day); Hcin: Normal rats received high dose of cinnamon (600 mg/kg/day).
* Number of rats in each group was 10.
** G6PDH was determined in blood.
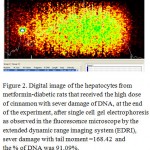 |
Figure 2: Digital image of the hepatocytes from metformin-diabetic rats.
|
Table 2. Effects of the low and high doses of cinnamon extract on serum levels of glucose and insulin in diabetic and metformin-diabetic rats.
| Groups | No. | Glu [mg/dL] | Insulin [ng/l] |
| DC | 10 | 136 ± 31 | 300 ± 72 |
| DM | 9 | 95.6a,b ± 17.2 | 222a ± 16 |
| DLcin | 10 | 101a ± 16 | 304 ± 74 |
| DHcin | 10 | 72.0b ± 15.3 | 208a ± 23 |
| DMLcin | 9 | 85.3a,b ± 11.1 | 187a ± 46 |
| DMHcin | 6 | 49.8c ± 11.19 | 168a ± 24 |
Values are presented as means with their standard deviations. Values not sharing a common superscript letter differ significantly at p>05 (one-way ANOVA and LSD-tests).
Glu: glucose.
DC: Diabetic control rats; DM: Metformin-diabetic rats; DLcin: Diabetic rats received low dose of cinnamon extract (300 mg/kg/day); DHcin: Diabetic rats received high dose of cinnamon extract (600 mg/kg/day); DMLcin: Diabetic rats treated with metformin and received low dose of cinnamon extract (300 mg/kg/day); DMHcin: Diabetic rats treated with metformin and received high dose of cinnamon extract (600 mg/kg/day).
a: Significantly different from DC; b: Significantly different from DM; c: Significantly different from DLcin; d: Significantly different from DHcin.
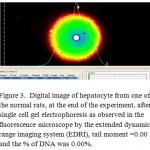 |
Figure 3: Digital image of hepatocyte from one of the normal rats.
|
Table 3: Effects of the low and high doses of cinnamon extract on serum levels of total cholesterol, high density lipoproteins-cholesterol, low density lipoproteins-cholesterol and triglycerides in diabetic and metformin-diabetic rats.
| Groups | No. | TC [mmol/L] | HDL-C [mmol/L] | LDL-C [mmol/L] |
| DC | 10 | 2.12a ± 0.32 | 0.380a ± 0.032 | 1.12a ± 0.11 |
| DM | 9 | 1.85b,c ± 0.12 | 0.417b ± 0.032 | 0.978a,b ± 0.164 |
| DLcin | 10 | 1.92a,b ± 0.17 | 0.400a,b ± 0.025 | 1.09a,b ± 0.11 |
| DHcin | 10 | 1.92a,b ± 0.34 | 0.460c ± 0.011 | 1.09a,b ± 0.24 |
| DMLcin | 9 | 1.79b,c ± 0.14 | 0.463c ± 0.018 | 1.05a,b ± 0.22 |
| DMHcin | 6 | 1.63c ± 0.30 | 0.470c ± 0.042 | 0.900b ± 0.21 |
Values are presented as means with their standard deviations. Values not sharing a common superscript letter differ significantly at p>05 (one-way ANOVA and LSD-tests).
TC: total cholesterol, HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol.
DC: Diabetic control rats; DM: Metformin-diabetic rats; DLcin: Diabetic rats received low dose of cinnamon extract (300 mg/kg/day); DHcin: Diabetic rats received high dose of cinnamon extract (600 mg/kg/day); DMLcin: Diabetic rats treated with metformin and received low dose of cinnamon extract (300 mg/kg/day); DMHcin: Diabetic rats treated with metformin and received high dose of cinnamon extract (600 mg/kg/day).
a: Significantly different from DC; b: Significantly different from DM; c: Significantly different from DLcin; d: Significantly different from DHcin.
Table 4: Effects of cinnamon extract and metformin administration on serum activities of glucose-6-phosphate dehydrogenase*, aspartate aminotransferase, alanine aminotransferase and lactate dehydrogenase in diabetic rats.
| Groups | No. | G6PDH [U/g Hb] | AST
[U/l] |
ALT
[U/l] |
LDH
[U/l] |
| DC | 10 | 9.67d ± 2.10 | 131a ± 16 | 62.0a ± 5.2 | 1359a,b ± 267 |
| DM | 9 | 12.1b,c ± 0.6 | 114b ± 9 | 50.0c,d ± 6.4 | 1071c ± 175 |
| DLcin | 10 | 10.8c,d ± 1.1 | 117b ± 10 | 54.3b,c ± 6.1 | 1245a,b,c ± 126 |
| DHcin | 10 | 11.1c ± 0.9 | 116b ± 12 | 45.7d ± 2.8 | 1159b,c ± 248 |
| DMLcin | 9 | 13.9a ± 1.6 | 112b ± 13 | 56.2a,b,c ± 3.9 | 1160b,c ± 261 |
| DMHcin | 6 | 13.2a,b ± 0.6 | 132a ± 19 | 57.3a,b ± 7.7 | 1494a ± 344 |
Values are presented as means with their standard deviations. Values not sharing a common superscript letter differ significantly at p>05 (one-way ANOVA and LSD-tests).
AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, and G6PDH: glucose-6-phosphate dehydrogenase.
DC: Diabetic control rats; DM: Metformin-diabetic rats; DLcin: Diabetic rats received low dose of cinnamon extract (300 mg/kg/day); DHcin: Diabetic rats received high dose of cinnamon extract (600 mg/kg/day); DMLcin: Diabetic rats treated with metformin and received low dose of cinnamon extract (300 mg/kg/day); DMHcin: Diabetic rats treated with metformin and received high dose of cinnamon extract (600 mg/kg/day).
*glucose-6-phsphate dehydrogenase was determined in blood.
a: Significantly different from DC; b: Significantly different from DM; c: Significantly different from DLcin; d: Significantly different from DHcin.
References
- Al Jamal, A. R. (2009) Effects of Cinnamon on Blood Glucose and Lipids Levels in Diabetic Patients (Type2), Jordan Journal of Biological Sciences, vol. 2(3): 135 – 138.
- Aleisa, A. M., Al-Rejaie, S. S., Bakheet, S. A., Al-Bekairi, A. M., Al-Shabanah, O. A., Al-Majed, A. H., Al-Yahya, A. A. and Qureshi S. (2008) Protective Effect of Metformin on Cardiac and Hepatic Toxicity Induced by Adriamycin in Swiss Albino Mice, Asian Journal of Biochemistry, vol. 3(2): 99-108.
- Anderson, R. A., Broadhurst, C. L., Polansky, M. M., Schmidt, W. F., Khan, A., Flanagan, V. P., Schoene, N. W. and Graves, D. J. (2004) Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity, Journal of agricultural and food chemistry,vol. 52(1): 65-70.
- Blasiak, J., Arabski, M., Krupa, R., Wozniak, K., Zadrozny, M., Kaszniski, J., Zurawska, M. and Drzwoski, J. (2004) DNA damage and repair in type 2 diabetes mellitus, Mutation, vol. 554: 297-304.
- Cao, H., Polansky, M. M. and Anderson, R. A. (2007) Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes, Archives of Biochemistry and Biophysics,vol. 459: 214-222.
- Castelli, W. P., Doyle, J. T., Gordon, T., Hames, C. G., Hjortland, M. C., Hulley, S. B., Kagan, A. and Zukel, W. J. (1977) HDL cholesterol and other lipids in coronary heart disease, Circulation, vol. 55: 767–772.
- Chavez, M. L., Jordan, M. A. and Chavez, P. I. (2006) Evidence- based drug-herbal interactions, Journal of Life Science, vol. 78: 2146-2157.
- Cryer, P. E. (2001) The prevention and correction of hypoglycemia. In Handbook of Physiology, Edited by: L.S. Jefferson and A.D. Cherrington, New York: Oxford University Press, 1057 –1092.
- Elassouli,S.M. Alqahtani,M.H. and Milaat, W. (2007) Geotoxicity of air borne particulates assessed by comet and the Salmonella mutagenicity test in Jeddah, Saudi Arabia, Int. J.Environ.Res.Public Health, vol.4:216-33.
- Ercal, N., Aykin-Burns, N., Gurer-Orhan, H. and McDonald, D. J. (2002) Oxidative stress in a phenylketonuria animal model, Free Radical Biology & Medicine, vol. 32(9): 906–911.
- Ernst, E. (2000) Possible interactions between synthetic and herbal medicinal products, Perfusion, vol. 13: 4-15.
- Ferrand-Drake, M., Friberg, H. and Wieloch, T. (1999) Mitochondrial permeability transition induced DNA-fragmentation in the rat hippocampus following hypoglycemia, Neuroscience, vol. 90(4): 1325-1338.
- Fugh-Berman, A. and Ernst, E. (2001) Herb-drug interactions: Review and assessment of report reliability, Blackwell Science Ltd Br J ClinPharmacol., vol. 52: 587-595.
- Gerstein, H. C., Miller, M. E., Byington, R. P., Goff, D. C. Jr, Bigger, J. T., Buse, J. B., Cushman, W. C., Genuth, S., Ismail-Beigi, F., Grimm, R. H., Jr, Probstfield, J. L., Simons-Morton, D. G. and Friedewald, W. T. (2008) Effects of Intensive Glucose Lowering in Type 2 Diabetes,The New England Journal of Medicine, vol. 358(24): 2545-2559.
- Gotto, A. M. (1988) Lipoprotein metabolism and the etiology of hyperlipedemia, Hospital practice,vol. 23(1): 4.
- Gupta, B. L., Nehal, M. and Baquer, N. Z. (1997) Effect of experimental diabetes on the activities of hexokinase, glucose-6-phosphate dehydrogenase and catecholamines in rat erythrocytes of different ages, Indian Journal of Experimental Biology, vol. 35: 792–795.
- Harkness, J. E. and Wagner, J. E. (1989) The biology and medicine of rabbits and rodents, Edited by: D.L. Coonnor, Philadelphia: Lee and Febiger.
- Jarvill-Taylor, K. J., Anderson, R. A. and Graves, D. J. (2001) A hydroxychalcone derived from cinnamon functions as a mimetic for insulin in 3T3-L1 diabetes, Journal of the American College of Nutrition, vol. 20: 327-336.
- Johansen,K. (1999) Efficacy of metformin in the treatment of NIDDM, Meta-analysis, Diabetes Care,vol. 22: 33-37.
- Kannappan, S., Jayaraman, T., Rajasekar, P., Ravichandran, M. K. and Anuradha, C. V. (2006) Cinnamon bark extract improves glucose metabolism and lipid profile in the fructose-fed rat, Singapore, vol. 47(10): 858-859.
- Khan, A., Safdar, M., Khan, M. M. L., Khattak, K. N. and Anderson, R. A. (2003) Cinnamon Improves Glucose and Lipids of People with Type 2 Diabetes, Diabetes Care, vol. 26: 3215-3218.
- Kim. S. H., Hyun, S. H. and Choung, S. Y. (2006) Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice, Journal of Ethnopharmacology, vol. 104(1-2): 119-123.
- Kornberg, A. and Horecker, B. L. (1955) Glucose-6-Phosphate Dehydrogenase. IN Methods in Enzymology,New York: Academic Press.
- Kunst, A., Draeger, B. and Ziegenhorn, J. (1983) UV-methods with hexokinase and glucose-6-phosphate dehydrogenase, Methods of Enzymatic Analysis, Deerfield, FL: VerlagChemie.
- Lohr, G. W. and Waller, H. D. (1974) Glucose-6-Phosphate Dehydrogenase. IN Methods of Enzymatic Analysis,New York: Academic Press.
- Mahan, L. K. and Escott-Stump, S. (2004) Krause’s food, nutrition and diet therapy, Elsevier.
- Marta, S., Maryse, P., and Nathalie, G. (2000) Effect of metformin on the vascular and glucose metabolic actions of insulin in hypertensive rats, American Journal of Physiology- Gastrointestinal and Liver Physiology, vol. 278: 682-692.
- Masiello, P., Broca, C., Gross, R., Roye, M., Manteghetti, M., Hillaire-Buys, D., Novelli, M. andRibes, G. (1998) Development of a new model of type 2 diabetes in adult rats administered with streptozotocin and nicotinamide. Diabetes, vol. 47:224.
- Navarro, C. M., Montilla, P. M., Martin, A., Jimenez, J. and Utrilla, P. M. (1993) Free radicals scavenger and antihepatotoxic activity of Rosmarinus, PlantaMedica, vol. 59: 312–314.
- Onaran, I., Guven, G. S., Ozdaş, S. B., Kanigur, G. and Vehid, S. (2006) Metformin does not prevent DNA damage in lymphocytes despite its antioxidant properties against cumenehydroperoxide-induced oxidative stress, Mutation Research/Genetic Toxicology and Environmental Mutagenesis, vol. 611(1-2): 1-8.
- Palmer, M. (2004) Dr. Melissa Palmer’s Guide to Hepatitis & Liver Disease: What You Need to Know, Avery.
- Qin, B., Nagasaki, M., Ren, M. and Al, E. (2003) Cinnamon extract (traditional herb) potentiates in vivo insulin-regulated glucose utilization via enhancing insulin signaling in rats, Diabetes Research and Clinical Practice, vol. 62: 139-148.
- Qin, B., Nagasaki, M., Ren, M., Bajotto, G., Oshida, Y. and Sato, Y. (2004) Cinnamon extract prevents the insulin resistance induced by a high-fructose diet, Hormone and Metabolic Research, vol. 36(2): 119-125.
- Saris, N. E. (1978) Revised IFCC method for aspartate aminotransferase, Clinical Chemistry, vol. 24: 720-721.
- Shirwaikar, A., Rajendran, K. and Kumar, D., C. (2004) Oral antidiabetic activity of Annonasquamosa leaf alcohol extract in NIDDM rats, Pharmaceutical Biology, vol. 42: 30-35.
- Singh, N. P. and Stephen, R. E. (1997) Microgel electrophoresis: sensitivity, mechanisms and DNA electrostretching, Mutation Research, vol. 383: 167–175.
- Solskov, L., Lofgren, B., Kristiansen, S. B., Jessen, N., Pold, R., Nelson, T., Boker, H. E., Schmitz, O. and Lund, S. (2008) Metformin induces cardioprotection against ischaemia/Reperfusion injury in the rat heart 24 hours after administration, Basic & Clinical Pharmacology & Toxicology, vol. 103(1): 82-87.
- Srividhya, S. and Anuradha, C. V. (2002) Metformin improves liver antioxidant potential in rats fed a high-fructose diet, Asia Pacific Journal of Clinical Nutrition, vol. 11: 319–322.
- Stadtman, T. C. (1957) Methods in Enzymology,New York: Academy Press.
- Suppapitiporn, S., Kanpaksi, N. and Suppapitiporn, S. (2006) The effect of cinnamon cassia powder in type 2 diabetes mellitus, Journal of The Medical Association of Thailand, vol. 89(3): 200-205.
- Theorell, T., Blomkvist, V., Lindh, G. and Evengard, B. (1999) Critical life events, infections, and symptoms during the year preceding chronic fatigue syndrome (CFS): an examination of CFS patients and subjects with a nonspecific life crisis, Psychosomatic Medicine, vol. 61: 304-310.
- Verspohl, E.J., Bauer, K. and Neddermann, E. (2005) Antidiabetic effect of Cinnamomum cassia and Cinnamomumzeylanicumin vivo and in vitro, Phytotherapy Research, vol. 19: 203-206.
- Wacker, W. E. C., Ulmer, D. D. and Vallee, B. L. (1956) Metalloenzymes and myocardial infraction: II Malic and lactic dehydrogenase activities and zinc concentration in serum, New England Journal of Medicine, vol. 255: 499.
- Wroblewski, F. and Ladue, J. S. (1956) Serum glutamic pyruvic transaminase in cardiac and hepatic disease, Proceedings of the Society for Experimental Biology and Medicine, vol. 91: 569-574.
- Zeevalk, G. D. and Nicklas, W. J. (2000) Lactate prevents the alterations in tissue amino acids, decline in ATP, and cell damage due to aglycemia in retina, Journal of Neurochemistry, vol. 75(3): 1027-1034.
- Ziegenfuss, T. N., Hofheins, J. E., Mendel, R. W., Landis, J. and Anderson, R. A. (2006) Effects of a Water-Soluble Cinnamon Extract on Body Composition and Features of the Metabolic Syndrome in Pre-Diabetic Men and Women, Journal of the International Society of Sports Nutrition,vol. 3(2): 45-53.
- Zlotogorski, H. A. and Littner, M. (2004) Potential risks, adverse effects and drug interactions associated with herbal medicine in dental patients, Refuat-Hapeh-Vehashinayim, vol. 21(2): 25-41.

This work is licensed under a Creative Commons Attribution 4.0 International License.





