How to Cite | Publication History | PlumX Article Matrix
Mohammad Sadeghi*, Nahid Ghasemi¹ and Mojgan Yarahmadi²
¹Department of Chemistry, Science Faculty, Islamic Azad University, Arak Branch, Arak Iran.
²Department of English, Faculty of Humanities, Arak Branch, Islamic Azad University, Arak Iran.
ABSTRACT: In this work, Large numbers of cyanide functional groups were introduced onto starch by grafting with polyacrylonitrile (PAN). The graft copolymerization reactions were carried out under nitrogen atmosphere using ceric ammonium nitrate (CAN) as an initiator. Evidence of grafting was obtained by comparing FTIR spectra and TGA of starch and the graft copolymer as well as solubility characteristics of the products. The synthetic conditions were systematically optimized through studying the influential factors including temperature, concentration of the initiator, acrylonitrile monomer and starch. Finally, Overall activation energy of grafting (Ea) was also estimated 39.7 kJ/mole.
KEYWORDS: Starch; Polyacrylonitrile; Graft ; Ceric ammonium nitrate; Copolymers
Download this article as:| Copy the following to cite this article: Sadeghi M, Ghasemi N, Yarahmadi M. Modification Structure of Starch by Graft Copolymerization with Poly(Acrylonitrile) and Calculation Grafting Parameters. Biosci Biotechnol Res Asia 2011;8(2) |
| Copy the following to cite this URL: Sadeghi M, Ghasemi N, Yarahmadi M. Modification Structure of Starch by Graft Copolymerization with Poly(Acrylonitrile) and Calculation Grafting Parameters. Biosci Biotechnol Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9213 |
Introduction
The modification of natural polymers is a promising method for the preparation of new materials. Graft copolymerization of vinyl monomers onto natural polymers is an efficient approach to achieve these materials1Superabsorbing resins were first developed with a view to utilizing agricultural materials, and are typed by the hydrolyzed corn starch-g-poly(acrylonitrile), H-SPAN. Since then, starches from different resources as well as other polysaccharides, for example, cellulose2, hydroxyethyl cellulose3, and guar gum were graft copolymerized to achieve water absorbing polymers. Polyacrylonitrile (PAN)4,polyacryamide, and poly(acrylic acid)5. have been frequently grafted, mostly onto starch, using different initiators especially the ceric-saccharide redox system6. Radical polymerization, however, has several disadvantages. The reproducibility of this method is poor, and there is little control over the grafting process, so the molecular weight distribution is polydisperse. In addition, the necessity for inert gases (e.g., argon) to prepare an oxygen-free atmosphere and the need for initiators, toxic and/or expensive monomers, and crosslinkers are other disadvantages of free-radical polymerization reactions. These problems have been reviewed in detail . For the first time6, with a new method, tried to synthesize of HSPAN superabsorbent hydrogel. They indicated by a solubility test that crosslinks were formed during graft copolymerization, by coupling of the two growing PAN radicals, and during saponification, by the attack of starch alkoxide ions on the nitrile groups as the initioation reaction of nitrile polymerization in the early stages of saponification. The nitrile groups of PAN were converted to a mixture of hydrophilic carboxamide and carboxylate groups during alkaline hydrolysis followed by in situ crosslinking of the grafted PAN chains. The initially formed oxygen–carbon bonds between the starch hydroxyls and nitrile groups of the PAN chains remained crosslinking sites. Then, Fanta et.al5 attempted to extend this idea to the preparation of superabsorbent hydrogels by the saponification of PAN in the presence of polyhydroxy polymers. Finally, reported the preparation of superabsorbing polymers from mixtures of PAN and various saccharides or alcohols.
Material and Methods
Materials
Starch (chemical grade, MW 50000) was purchased from Merck Chemical Co. (Germany). Ceric ammonium nitrate (CAN, Fluka) was used without purification. Acrylonitrile monomer (Merck) was distilled before use.
Prepare of initiator solution
The CAN solution was prepared by dissolving 2.74 g ceric ammonium nitrate in 50.0 mL of 1.0 N HNO3. Generally, 3.50 mL of this stock solution (0.10 M) was used for each experiment except for the experiments by which the effect of the initiator concentration was studied.
Graft copolymer preparation
A facial one step preparative method was used for synthesis of new copolymer based on St-polyacrylonitrile. A general procedure for produce of copolymer was conducted as follows. Starch (0.50-1.33 g) was added to a three-neck reactor equipped with a mechanical stirrer (Heidolph RZR 2021, three blade propeller type, 50-500 rpm), including 35 mL doubly distilled water. The reactor was immersed in a thermostated water bath. After complete dissolution of starch to form a homogeneous solution, a given volume of the above initiator solution was added to the starch solution at desired temperature (bath temperature, 35-75 oC). then the various amount of polyacrylonitrile (0.50-2.50 g) was dispersed in the reaction mixture to reaction for certain times (30-120 min). During the graft copolymerization, NH3 gas was evolved and a color change from red to light yellow. This discoloration was an indication of the reaction completion. Finally, After adding hydroquinon solution (0.5 wt%, 2 mL), cooling to room temperature, the product was then precipitated in excess amount of methanol while mild stirring for ten minutes. The product was filtered, thoroughly washed with methanol and dried at 50 oC for one hour 8.
Separation of homopolymer
To separate the polyacrylonitrile (PAN) homopolymer, 0.50 g of the crude product was poured in 50 mL of dimethylformamide (DMF) and stirred gently at 37 oC for 44 h. After centrifugating and decanting the supernatant (PAN in DMF), the starch-g-PAN was precipitated in methanol, thoroughly washed with methanol, and dried at 50 oC to reach a constant weight.
Infrared spectroscopy
FTIR spectra of samples were taken in KBr pellets using an ABB Bomem MB-100 FTIR spectrophotometer.
Results and Discussion
The simplest method to prove the formation of starch-g-Polyacrylonitrile is based on the solubility difference of the graft copolymer and the homopolymer, PAN. starch and PAN are soluble in water and DMF, respectively. When a reaction product was Soxhlet-extracted with DMF and alternately with water for 48h, an insoluble solid was still remained. A starch/PAN physical mixture was dissolved completely when it was treated in the same was. Therefore, it is obvious that the graft copolymer obtained was not a simple physical mixture, but some chemical bonds must exist between the starch substrate and PAN macromolecules 2-3.
The PAN grafting was also confirmed by the differences between FTIR spectra of the substrate and that of the graft copolymer. Figure 1 shows the FTIR spectra of the starch substrate, polyacrylonitrile (PAN) and the starch-g-PAN graft copolymer freed from homo Polyacrylonitrile. The existence of a sharp intense peak at 2242 cm-1 in IR spectra of the graft copolymers is a certain evidence of grafting. This absorption band arises from stretching vibration mode of the nitrile (CºN) groups. Most of the other peaks are related to the carbohydrate backbone. Since PAN could be extracted nearly completely from a physical mixture of PAN and polysaccharide by DMF, the presence of appreciable amounts of nitrile groups in our reaction products after extraction is an additional proof for grafting of polyacrylonitrile onto the polysaccharide.
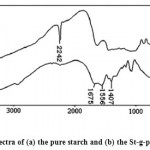 |
Figure 1: FTIR spectra of (a) the pure starch and (b) the St-g-poly(AN) copolymer.
|
Optimization of Polymerization
Since polymerization variables determine the extent of grafting and homopolymer amount, certain factors affecting the grafting parameters were investigated to achieve the optimum condition of polymerization9. Therefore, we optimized the grafting of acrylonitrile onto starch in homogenous aqueous media by changing temperature, the initial concentration of monomer, initiator, and the relative amount of the substrate. Within the range of the amount of the reactants used, our preliminary studies showed no considerable dependence between the reaction time and the grafting extent.
The conversion as well as the grafting parameters, i.e.homopolymer content (Hp), grafting ratio (Gr) and the grafting add-on values was calculated using the following equations:
Conversion= (W3-W0)/W1 …(1)
Gr= W3/W0 …(2)
Hp= W2/(W2+W3) …(3)
Add-on= (W3-W0)/W3 …(4)
Where W0, W1, W2 and W3 are weight of the initial substrate, the monomer charged, the homopolymer extracted, the homopolymer-free graft copolymer, and PAN side-chains separated, respectively.
Thermogravimetric Analysis
TGA curves for pure starch and starch-g-poly(AN) copolymer are shown in Figure 2. The grafted starch has shown improvement in thermal stability as clear from TGA curve. The initial decomposition temperature of the starch on grafting was increased from 172 to 413 oC with maximum decomposition rate at 567 oC, in comparison to original decomposition temperature of 335 oC of starch. These observations have clearly indicated that grafting of starch-g-poly(AN) copolymer has improved the thermal stability of starch12.
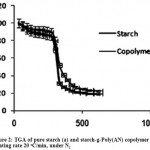 |
Figure 2: TGA of pure starch (a) and starch-g-Poly(AN) copolymer (b). Heating rate 20 oC/min, under N2.
|
Effect of Initiator Concentration
The grafting dependence on CAN concentration can be concluded from table1 and Figure 3 respectively. The highest grafting ratio (420%) was achieved at 0.004 mol/L of CAN where homopolymer content was 11.2%. Increased CAN concentration resulted in more radical sites on the polysaccharide backbone that in turn led to higher Gr and add-on values and lower homopolymer formation 13-14. However, since the CAN initiator solution is used as dilute HNO3, at CAN concentration higher than 0.004 mol/L, a more acidic pH probably causes partially termination of the macroradicals on starch. As a result, increased free radicals on starch are compensated by partial termination of the macroradicals. Thus Gr and add-on values were diminished at higher amounts of the initiator13.
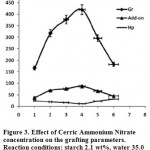 |
Figure 3: Effect of Cerric Ammonium Nitrate concentration on the grafting parameters. Reaction conditions: starch 2.1 wt%, water 35.0 mL, AN 0.8 molL-1, temperature 45 °C. |
Table 1: Grafting parameters from the graft polymerization of arcrylonitrile onto starch at different amount of the initiator.
| CAN, mol/L | Conversion, % | Gr% | Hp% | Add-on% |
| 0.001 | 21.4 | 166.7 | 24.3 | 37.0 |
| 0.002 | 42.5 | 317.8 | 19.7 | 68.8 |
| 0.003 | 64.8 | 377.3 | 16.1 | 76.4 |
| 0.004 | 81.9 | 420.0 | 11.2 | 87.1 |
| 0.005 | 58.3 | 296.0 | 22.4 | 66.7 |
| 0.006 | 10.3 | 182.2 | 31.7 | 44.9 |
Effect of Monomer Concentration
The effect of monomer amount on the grafting reaction was studied at various concentrations of AN while other influential factors were unchanged. The grafting parameter variations are changed by the amount of charged monomer (Table 2). The grafting extent is significantly increased due to more availability of monomer for grafting. However,as showed in figure 4, beyond a certain Gr value, i.e., 418% at 0.5 molL-1 AN, the trend is inversed. The conversion and the Add-on are decreased, and homopolymer content is increased noticeably from 14.4 to 32.9 percent. Thus, acrylonitrile in an amount of 1.8 g (0.5 mol
L-1) was recognized as an optimum monomer concentration. Once the monomer units are added, an excess of monomer can only increase the optimum volume of the reaction mixture. The resulting reduced relative concentration of the initiator and substrate leads to decreased conversion and grafting efficiency15.
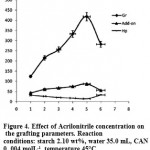 |
Figure 4: Effect of Acrilonitrile concentration on the grafting parameters. Reaction conditions: starch 2.10 wt%, water 35.0 mL, CAN 0. 004 molL-1, temperature 45 °C. |
Table 2: Grafting parameters from the graft polymerization of arcrylonitrile onto starch at different amount of the monomer.
| Acrylonitrile, molL-1 | Conversion, % | Gr% | Hp% | Add-on% |
| 0. 1 | 22.8 | 123.8 | 31.3 | 43.1 |
| 0. 2 | 49.0 | 215.0 | 26.4 | 60.8 |
| 0. 3 | 66.1 | 256.7 | 20.2 | 67.2 |
| 0. 4 | 73.5 | 333.3 | 17.2 | 74.7 |
| 0.5 | 88.8 | 418.0 | 14.4 | 87.3 |
| 0.6 | 44.7 | 282.2 | 32.9 | 55.8 |
Effect of Substrate Concentration
The related to the grafting dependence on starch amount is summarized in Table 3. As showed in figure 5, maximum grafting and the lowest homoPAN formation was observed at 1.7 g (3.0 wt%) starch, while others reactants including, monomer, initiator, and temperature were kept constant. Beyond this value, both grafting ratio and add-on values are considerably reduced17. this behavior is attributed to the availability of more grafting sites for initiation of graft copolymerization at higher concentration of the substrate (from 1.0 to 3.0 wt% starch). However, upon further increase in the substrate concentration, increase in the reaction medium viscosity restricts the movements of macroradicals leading to decreased grafting ratio and add-on values16. It also may be attributed to deactivation of the macroradical growing chains (e.g., by transfer reactions, combination and/or interaction with the primary radicals) soon after their formation18.
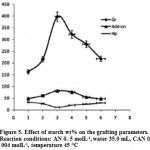 |
Figure 5: Effect of starch wt% on the grafting parameters. Reaction conditions: AN 0. 5 molL-1,water 35.0 mL, CAN 0. 004 molL-1, temperature 45 °C. |
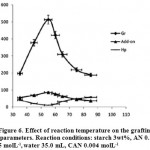 |
Figure 6: Effect of reaction temperature on the grafting parameters. Reaction conditions: starch 3wt%, AN 0. 5 molL-1,water 35.0 mL, CAN 0.004 molL-1. |
Table 3: Grafting parameters from the graft polymerization of arcrylonitrile onto starch at different concentration of the substrate.
| Starch, wt% | Conversion, % | Gr% | Hp% | Add-on% |
| 1.0 | 37.8 | 162.2 | 33.1 | 48.4 |
| 2.0 | 44.9 | 216.7 | 27 | 61.6 |
| 3.0 | 86.3 | 397 | 11.6 | 81.7 |
| 4.0 | 58.2 | 322 | 20.5 | 76.7 |
| 5.0 | 48 | 280 | 24.8 | 55.2 |
| 6.0 | 41.7 2 | 17.8 | 29.9 | 47.8 |
Effect of Temperature
To study the influence of the reaction bath temperature on the grafting parameters, the grafting of AN onto starch was carried out at six temperature ranging from 35 to 75 oC. The results are given in Table 4. As showed in figure 6, Grafting percentage (%Gr) is increased with increasing the temperature from 35 to 55 oC, and then decreased. At 55 oC, maximum grafting (Gr 513.7%), minimum homopolymer content (11.5%) and highest add-on value (86.3%) was obtained. Improvement of grafting up to 55 oC can be attributed to the following factors: increased the number of free radicals formed on the starch backbone, increased propagation of the graft copolymerization onto starch, enhanced diffusion of monomer and initiator into and onto backbone structure, and increased in mobility of the monomer molecules and their higher collision probability with the backbone macroradicals19. However, Gr was decreased as the bath temperature was raised beyond 55 oC. This can be accounted for in terms of chain radical termination at higher temperature17. Premature termination of growing chains and instability of the ceric-saccharide complex (5) are presumably another reasons for reduced amount of grafting beyond 55 oC. The PAN homopolymer formation is minimal at the bath temperature of 55 oC18.
Table 4. Grafting parameters from the graft polymerization of arcrylonitrile onto starch at different temperatures.
| Temperature, °C | Conversion, % | Gr% | Hp% | Add-on% |
| 35 | 24.5 | 197.3 | 41.1 | 55.3 |
| 45 | 46.7 | 377.8 | 21.6 | 69.5 |
| 55 | 81.8 | 513.7 | 11.5 | 86.3 |
| 60 | 59.6 | 422.2 | 17.4 | 76.4 |
| 65 | 41.3 | 309.8 | 33.1 | 63.7 |
| 75 | 18.7 | 218.7 | 48.6 | 45.6 |
Grafting Mechanism and Rate
It has been shown that the anhydroglucose units are predominantly oxidized through C2-C3 bond cleavage induced by Ce4+ ions. Therefore, a general reaction scheme for grafting, in analogy with that previously proposed may be as follows (Scheme 1).
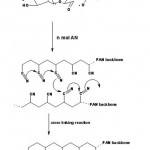 |
Scheme 1: Proposed mechanism for graft copolymer of acrylonitrile on to starch. |
The reactive vicinal OH groups form a complex with ceric ion. The complex may dissociate, giving rise to free radical sites onto the polysaccharide backbone and these radicals initiate the graft polymerization.
The rate of grafting (Rg) may be evaluated as measures of the rate of monomer disappearance by using the following expression20.
Rg (mol L-1s-1) = 1000W3/MTV …(5)
M (gmol-1) is the molecular weight of the monomer. T and V stand for total reaction time (s) and total volume (mL) of the reaction mixture21-22.
Overall activation energy of grafting (Ea) may also be estimated from the temperature data through plotting lnRg versus 1/T (oK-1) for the initial portion of the data of the temperature series given in Table 4. The slope of this Arrhenius plot (Figure 7) resulted in a rough estimation of Ea of grafting using the relationship slope = -Ea/R; where R is the universal gas constant. Therefore, Ea was found to be 39.7 kJ/mole (9.5 kcal/mole).
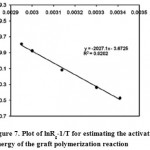 |
Figure 7: Plot of lnRg-1/T for estimating the activation energy of the graft polymerization reaction. |
Conclusion
A doubly modified cellulose, St-g-polyacrylonitrile, was prepared using ceric-initiated graft polymerization of acrylonitrile (AN) onto starch. The study of FTIR spectra and thermogravimetric analysis provide that the graft copolymerization takes place. the synthetic conditions were systematically optimized through studying the influential factors including temperature, concentration of the initiator, the monomer AN, and the substrate. The effect of the individual factors was investigated by calculating the grafting parameters, i.e., grafting ratio (Gr), add-on value, homopolymer content (Hp), and conversion. Under optimum conditions (starch 3.0 wt%, AN 0. 5 molL-1, CAN 0.004 molL-1, reaction bath temperature 55 oC, reaction time 2h), the grafting parameters were achieved as 513%, 86.3%, 11.5%, and 81.8%, respectively. The hydrophobically modified starch exhibited higher thermal stability in comparison with non-modified starch. The PAN-grafted starch may be a candidate for manufacture of molded plastics, ion exchange resins, and plastic films and in cosmetics.
References
- F. L. Buchholz and A. T. Graham, in: Modern Superabsorbent Polymer Technology. Wiley, New York(1997).
- A. S. Hoffmanin: Polymeric Materials Encyclopedia. J.C. Salamone (Ed.), Vol. 5, p. 3282. CRC Press, Boca Raton, Florida, (1996).
- N. A. Peppas and A. G. Mikesin: Hydrogels in Medicine and Pharmacy. Vol. 1, CRC Press, Boca Raton, Florida(1986).
- Heinze, T.; Liebert, T. Unconventional Methods in Cellulose Functionalization. Prog. Polym. Sci.26: 1689-1762 (2001).
- Fanta, G.F., Doane W.M. In Modified Starches: Properties and Uses; Wurzburg, O.B., Ed.; CRC Press: Boca Raton (Florida), 149-178 (1986).
- Athawale, V.D.; Rathi, S.C.Func. Polym.34: 11-17 (1997).
- Sandle, N.K.; Verma, O.P.S. Varma, I.K. Thermochim. Acta, 115: 189-198 (1987).
- Sadeghi,M; Hosseinzadeh, H;J.Chil.Soc., 55: N04(2010)
- Sadeghi,M; Hosseinzadeh, H; Turkish Journal of Chemistry, 34: 739-752 (2010)
- thawale, V.D.; Rathi, S.C. J. Macromol. Sci.-Rev. Macromol. Chem. Phys. C39(3): 445-480 (1999).
- Hebeish, A.; Guthrie, J.T. The Chemistry and Technology of Cellulosic Copolymers, Springer: Berlin (1981).
- Pourjavadi, A.; Zohuriaan-Mehr, M.J, Starch/Starke.,54: 140-147 (2002).
- Okieimen, F.E.; Ogbeifun, D.E. Eur. Polym. J.32(3): 311-315 (1996).
- Sadeghi,M; Hosseinzadeh, H; Journal of applied polymer science, 108: 1142-1151 (2008).
- Okieimen, F.E. J. Appl. Polym. Sci. 89: 913-923 (2003).
- Leza, M.L.; Casinos, I.; Guzman, G.M. Eur. Polym. J.25(12): 1193-1196 (1989).
- Leza, M.L.; Casinos, I.; Guzman, G.M. Brit. Polym. J. 23: 341-346 (1990).
- Zhang, L.-M.; Tan, Y.-B. Eng. 280/281: 59-65 (2000).
- Hsu S.C, Don T.M, Chiu W.Y, PolymDegrad Stab; 75: 73 (2002).
- Zohuriaan, M. J; Pourjavadi, A; Sadeghi, M, iranian polymer journal., 14(2): 131-138 (2005).
- Tan, Y.; Zhang, L.; Li, Z. J. Appl. Polym. Sci. 69: 879-885 (1998).
- Zhang, J.; Zhang, L.-M.; Li, Z.-M. J. Appl. Polym. Sci. 78: 537-542 (2000).

This work is licensed under a Creative Commons Attribution 4.0 International License.





