Manuscript accepted on : October 08, 2011
Published online on: 28-12-2011
Comparative Morphology of the Otolith
A. Mansourkiaei¹, T. Valinasab², G. H. Vosoughi³ and P. Ghavam Mostafavi³
¹Department of Marine Biology, Tonekabon Branch, Islamic Azad University, Tonekabon Iran.
²Iranian Fisheries Research Organization, P.O.Box: 14155-6116, Tehran Iran.
³Department of Marine Biology, Science and Research branch, Islamic Azad University, Tehran Iran.
Corresponding Author e-mail: ana_kiaei@yahoo.com
ABSTRACT: The otolith morphology of 10 species belonging to the Carangidae family collected from the Persian Gulf and Oman Sea (coast of Iran). Were examined separately and the characteristics were drawn. The morphomttric parameters determined were total length (TL, mm), weight (W, gr), otolith length (OL, mm), weight of right otolith(WRO, mm), weight of left otolith(WLO, mm), height of right otolith (HRO, mm), height of left otolith (HLO, mm),. The values obtained from measurements are given in the 90% confidence interval in most spesies. The observation of this family three shape of sagittal, Sagitiform, Fusiform and Lanceolated. As a result of this analysis, it is possible to identify the species from the Carangidae family by the otolith characters.
KEYWORDS: Otolith; Morphology; Sagitta; Carangidae; Persian Gulf; Oman Sea
Download this article as:| Copy the following to cite this article: Mansourkiaei A, Valinasab T, Vosoughi G. H, Mostafavi P. G. Comparative Morphology of the Otolith. Biosci Biotechnol Res Asia 2011;8(2) |
| Copy the following to cite this URL: Mansourkiaei A, Valinasab T, Vosoughi G. H, Mostafavi P. G. Comparative Morphology of the Otolith. Biosci Biotechnol Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9226 |
Introduction
Otoliths are acellular concretions of calcium carbonate and other inorganic salts, witch develop over a protein matrix in the inner ear of vertebrates, in close association with the sensitive maculae of labyrinthic compartments (Weichert and Prech 1981; Hildebrand, 1988; Jobling, 1995). Otoliths are enclosed in three compartments linked with the ear in teleost fishes (Popper et al, 2005). The labyrinth includes three semicircular canals oriented in different planes and three compartments: the utriculus, sacculus and lagena. Each compartment contains otoliths (earbones or earstones), the lapillus, sagitta,and asteriscus (Berra &Aday,2004).The sacular otolith (sagitta) is the largest and the utricular otolith (lapillus) is the smallest among the three (Paxton, 2000) at least in most teleost families (Schulzmirbach and Reichenbacher, 2006). Although the morphological features of otoliths are highly variable between species, ranging from the relatively simple disc shape of some flat fishes (Pleuronectidae) to the irregular shape of others, a high level of species specificity has, for along time, been used to achieve various taxonomic objective (Hecth, 1987; Hunt, 1992).
Otoliths have an important biological function because they enable the inner ear to mediate the senses of hearing and balance (Popper et al, 2005). Otoliths growth is related to increase in size of the fish and generally follows and allometric increase in dimensions (Chilton and Beanish, 1982).
In addation to the use of otoliths for estimating age of fish, they may also be used to characterize stock specific differences or to interpolate size at age based on some relation between otolith and fish dimension (Hunt, 1992).Numerous studies have been undertaken to estimate size at some earlier age (back-calculation) based on relationships between otolith dimension and fish size.
Otoliths of each species of fish have characteristic shapes and features and given adequate comparative material or appropriate keys, identification to species can usually be done provided that the otoliths are not broken or badly digested. The fact that otoliths persist in the stomach, intestines, or feces after after soft parts and bones have disappeared increases their utility.
In the present work, an attempt was made to describe the otolith morphological characters of the family Carangidae collected mainly from Persian Gulf and Oman Sea. There is no work or record that deals solely with the otoliths of carangidae in Persian Gulf and Oman Sea. The aim of this study was to provide new information regarding otolith morphology and body size relationships of 10 species of carangidae.
Materials and methods
The otolith of 10 species of Persian Gulf and Oman Sea carangidae were examined. The number of observations on each species and range in fish lengths is given in table 1. The total length and body weight in fishes were measured.
Table 1: Sample sizes of 10 carangid species in the Persian Gulf and Oman Sea.
| Species | N | Standard length range (mm) | Mean Standard length (mm) | Weight range (gr) | Mean weight (gr) |
| Parastromateus niger | 59 | 130-370 | 173.02±64.67 | 98-2051 | 320.14±507.54 |
| Alectis indicus | 30 | 155-490 | 319.67±79.14 | 147.24-2000 | 819.53±383.73 |
| Uraspis helvola | 30 | 145-290 | 185.43±36.62 | 109.67-287.67 | 194.40±50.37 |
| Atropus atropus | 42 | 140-200 | 160.14±16.14 | 94.46-260.4 | 153.32±45.29 |
| Megalaspis cordyla | 26 | 260-380 | 292.50±39.93 | 263.12-673.07 | 359.99±146.28 |
| Scomberoides commersonnianus | 29 | 292-425 | 334.10±28.84 | 205.73-777.27 | 518.20±127.84 |
| Caranx papuensis | 27 | 152-245 | 176.33±17.11 | 95.57-388.14 | 167.68±52.58 |
| Aleps djedaba | 64 | 155-190 | 169.56±9.49 | 76.2-165.35 | 112.05±18.61 |
| Carangoides chrysophrys | 32 | 153-362 | 195.69±42.13 | 97.27-544.45 | 214.98±111.00 |
| Carangoides armatus | 30 | 143-235 | 186.93±18.23 | 126.76-555.26 | 282.15±78.91 |
Fish were caught in the Persian Gulf and Oman Sea by trawling ship. The total length and weight, for each fish were determined. Only sagittal otoliths were extracted from fresh specimens. These otoliths are located on the two sides of basioccipital bone and are separated by a thin septum arising from the mid ventral ridge of the occipital (Ruck, 1976). The otoliths were removed by turning the ventral side of the fish upward to allow removal of the lower jaw, the gills and the hypobranchial apparatus and to expose the base of skull. With a sharp scalpel, the optic capsules were separated and the otoliths gently removed with a pair of fine tweezers. Later, the otoliths were cleaned with 70% ethanol and stored dry in small glass tube.
Length (OL), defined as the longest dimension between the anterior and posterior edges of the otolith, and Otolith Height (OH) as the dimension from the dorsal to ventral edge, and Antirostrum Length of Otolith (LARO), and Antirostrum Height of Otolith (HARO), and Rostrum Length of Otolith(LRO),and Rostrum Height of Otolith(HRO)(Fig. 1).
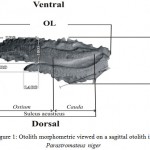 |
Figure 1: Otolith morphometric viewed on a sagittal otolith in Parastromateus niger.
|
Length (OL), defined as the longest dimension between the anterior and posterior edges of the otolith, and Otolith Height (OH) as the dimension from the dorsal to ventral edge, and Antirostrum Length of Otolith (LARO), and Antirostrum Height of Otolith (HARO), and Rostrum Length of Otolith(LRO),and Rostrum Height of Otolith(HRO)(Fig. 1).
The sagittal otolith of each species, both left and right, photographs taken by scaning electron microscope (Philips XL30). The significance of the variance one way (ANOVA) relationships between otolith and fish length and fish weight. Difference between right and left sagittae were tested using a paired t-test. The following morphometric relationships were analyzed.
Results
All parameter measured show significant morphometric between left and right otolith, (Table 2). The shape of the otolith in carangidae was different and can be classified into three types: fusiform, sagitiform, lanceolate and variable of margin in otolith were determined (Table 2).
Table 2: Shape of otolith and different mode opening and kinds of denticulate
| Species | Sagittal forms | Mode oppening | Mode position | kinds of Ventral margin denticulate | kinds of Dorsal margin denticulate |
| Parastromateus niger | Fusiform | Ostial | Supramedian | Crenate | Irregular |
| Alectis indicus | Sagitiform | Ostial | Supramedian | Irregular, Dentate,Crenate | Irregular, Dentate, Crenate |
| Uraspis helvola | Sagitiform | Ostial | Median | Crenate, Entire | Entire,Crenate,Irregular |
| Atropus atropus | Fusiform | Ostial | Supramedian | Crenate | Irregular, Crenate |
| Megalaspis cordyla | Lanceolated | Ostial | Median | Crenate | Dentate |
| Scomberoides commersonnianus | Sagitiform | Ostial | Median | Crenate, Dentate | Irregular, Dentate |
| Caranx papuensis | Fusiform | Ostial | Supramedian | Crenate | Sinuate |
| Aleps djedaba | Fusiform | Ostial | Median | Sinuate,Crenate | Sinuate,Crenate |
| Carangoides chrysophrys | Fusiform | Ostial | Median | Crenate | Crenate, Sinuate |
| Carangoides armatus | Fusiform | Ostial | Median | Crenate | Sinuate, Crenate |
The relationships of otolith length and otolith weight with fish length and fish weight in all species was observed. Generally, standard length of fishes is linearly related to otolith length. Otolith length typically is linearly related to length of fish until the fish reaches maximum size; thereafter, the otolith increase only in thickness.
Table 3: Differences between right and left otolith of 10 carangid species from the Persian Gulf and Oman Sea. (OL) otolith length, (OW) otolith weight, (OH) otolith height (N=number of right otolith + left otolith).
| Species | N | parameter | t | Df | P (α=0.05) |
| Parastromateus niger | 118 | OL
OW OH |
4.05
1.005 1.564 |
117 | 0.31
0.02 0.29 |
| Alectis indicus | 58 | OL
OW OH |
2.564
0.458 0.254 |
57 | 0.16
0.21 0.02 |
| Uraspis helvola | 52 | OL
OW OH |
4.564
0.564 0.458 |
51 | 0.32
0.32 0.01 |
| Atropus atropus | 62 | OL
OW OH |
3.154
0.642 0.304 |
61 | 0.14
0.12 0.10 |
| Megalaspis cordyla | 54 | OL
OW OH |
3.256
0.986 1.542 |
53 | 0.26
0.01 0.10 |
| Scomberoides commersonnianus | 60 | OL
OW OH |
4.293
0.237 1.569 |
59 | 0.26
0.25 0.14 |
| Caranx papuensis | 60 | OL
OW OH |
3.045
1.084 0.987 |
59 | 0.00
0.10 0.25 |
| Aleps djedaba | 84 | OL
OW OH |
6.254
2.356 0.897 |
83 | 0.14
0.10 0.14 |
| Carangoides chrysophrys | 128 | OL
OW OH |
2.564
0.804 3.042 |
127 | 0.02
0.16 0.12 |
| Carangoides armatus | 60 | OL
OW OH |
2.609
1.015 0.458 |
59
|
0.29
0.04 0.21 |
Analyses of otolith morphometric parameters vs.TL in the some species were showed high correlation and the some species were showed less correlation (Table 4). The relationship between fish OW and total length, the coefficient of determination being higher than 0.88 in all species (Table 4).
Table 4: Relationship between otolith morphometric parameters and Total length (TL). Coefficient of determination (R). OL: otolith length, OW: otolith height and O width. All regressions were statistically significant at P< 0.05.
| Species | N | OL(R). TL | R | O Weight(R). TL | R | O height(R). TL | R |
| Parastromateus niger | 59 | TL=0.1213OL + 2.8803 | 0.8032 | W=0.00000005 TL 1.4096 | 0.9158 | TL = 0.042 W + 1.4562 | 0.759 |
| Alectis indicus | 29 | TL =0.0804OL+ 3.0699 | 0.3122 | W = 0.00005 TL 1.654 | 0.9643 | TL=0.0309 W + 0.8184 | 0.3475 |
| Uraspis helvola | 26 | TL=0.1739OL+ 0.2737 | 0.8716 | W = 0.00016 TL 1.554 | 0.9784 | TL=0.0708 W + 0.8474 | 0.4586 |
| Atropus atropus | 31 | TL=0.0872OL + 3.2685 | 0.519 | W = 0.000042 TL 1.454 | 0.9452 | TL=0.0658 W + 0.8478 | 0.658 |
| Megalaspis cordyla | 27 | TL=0.1302OL + 2.2424 | 0.3793 | W = 0.000047 TL 1.524 | 0.9684 | TL=0.0356 W + 0.8564 | 0.7458 |
| Scomberoides commersonnianus | 30 | TL=0.1934OL + 0.8034 | 0.6513 | W = 0.00034 TL 1.425 | 0.9584 | TL=0.0547 W + 0.8147 | 0.458 |
| Caranx papuensis | 30 | TL=0.167 OL + 0.6618 | 0.8788 | W = 0.000095 TL 1.475 | 0.8874 | TL=0.0987 W + 0.8478 | 0.652 |
| Aleps djedaba | 42 | TL=0.1327OL + 1.1423 | 0.5646 | W = 0.00042 TL 1.356 | 0.9025 | TL=0.0487 W + 0.8256 | 0.485 |
| Carangoides chrysophrys | 64 | TL = 0.112OL+ 2.3253 | 0.3684 | W = 0.00036 TL 1.428 | 0.9741 | TL=0.0358 W + 0.8745 | 0.6857 |
| Carangoides armatus | 30 | TL=0.1228 OL+ 1.5073 | 0.8538 | W = 0.000084 TL 1.358 | 0.9857 | TL=0.0458 W + 0.8658 | 0.3284 |
| Species | N | OL (L). TL | R | O Weight (L). TL | R | O Height (L). TL | R |
| Parastromateus niger | 59 | TL=0.1233OL + 2.8608 | 0.8188 | W = 0.0000008 TL 1.3046 | 0.9058 | TL = 0.061 W + 1.2652 | 0.8171 |
| Alectis indicus | 29 | TL=0.0824OL + 3.0879 | 0.3022 | W = 0.00018 TL 1.589 | 0.9433 | TL=0.0819 W + 0.8354 | 0.3547 |
| Uraspis helvola | 26 | TL=0.1439 OL+ 0.2537 | 0.8416 | W = 0.00016 TL 1.487 | 0.9844 | TL=0.0678 W + 0.8474 | 0.4458 |
| Atropus atropus | 31 | TL=0.1042OL + 3.2245 | 0.6014 | W = 0.000027 TL 1.467 | 0.9012 | TL=0.0988 W + 0.8475 | 0.6141 |
| Megalaspis cordyla | 27 | TL=0.1812 OL+ 2.1725 | 0.3283 | W = 0.000057 TL 1.547 | 0.9648 | TL=0.0428 W + 0.8624 | 0.7464 |
| Scomberoides commersonnianus | 30 | TL =0.2134OL+ 0.9434 | 0.6054 | W = 0.00041 TL 1.414 | 0.9628 | TL=0.0604 W + 0.8247 | 0.4628 |
| Caranx papuensis | 30 | TL=0.185 OL + 0.6485 | 0.8958 | W = 0.000091 TL 1.423 | 0.8947 | TL=0.0969 W + 0.8592 | 0.7014 |
| Aleps djedaba | 42 | TL=0.1542OL + 1.1628 | 0.5847 | W = 0.00043 TL 1.361 | 0.9104 | TL =0.04684W+ 0.8256 | 0.4924 |
| Carangoides chrysophrys | 64 | TL =0.1042OL+ 2.3451 | 0.3617 | W = 0.00034 TL 1.431 | 0.9801 | TL = 0.0406W+ 0.8799 | 0.6918 |
| Carangoides armatus | 30 | TL=0.1087OL + 1.5847 | 0.8478 | W = 0.000087 TL 1.362 | 0.9804 | TL=0.04628W + 0.9014 | 0.3452 |
Discussion
The present investigation has shown that the specific morphology of the otolith can used the taxonomy and identification in carangidae. All equations relating otolith length with fish size proportion of the variance in the all species.
Otoliths of each species of fish have characteristic shapes and features and given adequate comparative material or appropriate keys, identification to species can usually be done provided that the otoliths are not broken or badly digested(Frost, 1981).
Relationship between otolith morphometric parameters and fish total length and weight for carangidae species studied were observed. Generally, total length of fishes is linearly related to otolith length. Otolith length typically is linearly related to length of the all species examined (Table 4). Newman (2002) and Mosegaard & Reeves (2001) have recorded a linear relationship for both the total body length and weight and otolith weight. Al Dubakel in 2006 reported the relationships between both fish body size versus weight of the oto lith, eye lens and liver were studied in Acanthopagrus latus Therapon theraps, and Pelates quadrilineatus collected from the Khor Al-Zubair area, Iraq
Otolith lengths of larval and juvenile fishes may increase in a curvilinear fashion relative to fish length for some species, such as sockeye salmon (Oncorhynchus nerka ; West and Larkin, 1987) . The relationship between otolith length and fish length may be dependent on the growth rate of the fish, as was reported for striped bass (Morone saxatilis; Secor and Dean, 1989).Similar results have been reported for many fish species(Jawad, 2007; Hunt, 1992; Volpedo et al, 2006).
Studies of sagitta otolith morphometric parameter in all species of carangidae in research significantly between right and left otolith and different in size, similar to in a rockfish species left and right sagitta also may differ in size (Wyllie, 1987).Although in 8 species of Atlantic Ocean fishes were carried out and were not significantly different between left and right otolith (Hunt, 1992). Investigation of sagitta otolith morphometric parameter in 4 species of sciaenidae did not show significant morphometric differences between left and right otoliths, only otolith width in white mouth croaker and otolith length in king weakfish showed significant differences between left and right otoliths (Waessle et al., 2003).
Analyzing the morphometric relationships, we concluded that otolith length and otolith weight are indicators of fish total length and fish weight in all species. In most species otolith length and fish length the potential regression explained more than 90% variation and in most species otolith weight and fish weight 90% variation. Baldas et al (1997) described the relationship between otolith length and fish total length by using potential models in stripped weakfish (50-600mmTL) and linear models in Whitemouth croaker ( 140-370mm TL). Also Waessle et al (2003) observed similar to results in juvenile sciaenidae.
Otolith growth is generally thought to uncouple from somatic growth at a very early age (Munk and smikrud, 2001). A variety of factors influence the degree or timing of this uncoupling (Moksness et al, 1995).
In this study has shown that the specific morphology of the otoliths examined can be used for taxonomic identification of carangidae species. The observation of this family three shape of sagittal, Sagitiform, Fusiform and Lanceolated (Table 2).
The analysis between left and right otolith showed morphometric difference. Sagitta is the best otolith to recognize in carangidae.
Acknowledgments
We thanks Dr. Mohammad Reza Fatemi for his guides in research and we would like to thanks Mr. Azhir for collected in samples from trawl ship. The author thanks the director Mr Pourbakhshian and the staff of laboratory in Islamic Azad university Tonekabon branch.
References
- Al-Dubakel, A.Y & J. N. Abdullah. 2006. Relationship of body size with some body structures of three young marine fish species collected from Khor Al-Zubair, Iraq, Anales de biologia 28:95-99
- Baldas, M.I., G. Perez Macri, A.V. Volpedo and D.D. Echeverria. 1997. Morphology sagittal in Carangidae, Scianidae, Mullidae in costal sea of Argentina, 19: 99-112.
- Berra, T. M. & Aday, D. D., 2004. Otolith description and age-and-growth of Kurtus gulliveri from northern Australia. J. Fish Biol. 65, 354–362.
- Chilton, D.E., and R.J. Beamish, 1982. Age determination methods for studies by the Ground fish program at the pacific Biological station. Can. Spec. Pub. Fish. Aquatic Sci, 60: 102 P
- Frost, K. J. & Lowry, L. F., 1981. Trophic importance of some marine gadids in northern Alaska and their body-otolith size relationships, Fish. Bull., 79, 187-192.
- Hecht T. 1987. Guide to the otoliths of southern Ocean fishes. Suid-Afrikaanse Tydskrif Vir Antarkieses Navorsing 17: 2-87.
- Hunt, J.J. 1992. Morphological characteristics of otoliths for selected fish in the Northwest Atlantic, Journal Northw Atl. Fish.Sci., (13):63-75.
- Jawad, L.A.,Al-Jufaili, S.A, Al-Shuhaily, S.S. Morphology of the otolith of the greater Lizardfish Saurdiatumbil (pisces:synodontidae), Journal of Natural History, 42.(35-36):2321-233. 2008.
- Jobling, M. and A. B Reiby. 1986. The use and abuse of fish otoliths in studies of feeding habits of marine piscivorores, Sarsia, 71:265-274.
- Moksness, E., K. Rukan, L. Ystanes, A. Folkvord, and A. Johannessen. 1995. Comparison of somatic and otolith growth in North sea herring (Clupea harengus L.) larvae: evaluation of growth dynamics in mesocosms. University of South Carolina Press, Columbia. P: 119-134
- Mosegaard H & Reeves SA. 2001. Revision of Baltic cod age determination based on otolith accretion charac- teristics and weight distribution. International Council for the Exploration of the Sea. Report 2001/p12
- Munk, K. M. 2001. Maximum ages of ground fishes in waters off Alaska and British Columbia and considerations of age determination. Alaska Fishery Research Bulletin 8(1): 12-21.
- Newman SJ. 2002. Age, growth, mortality, and population characteristic of the pearl perch (Glaucosoma buer– geri Richardson, 1845), from deeper continental shelf waters off the Pibara coast of North Western Australia. Journal of Applied Ichthyology 18 (2): 95-101.
- Paxton, J.R.Fish otoliths: do size correlate with taxonomic group, habitat and Luminescence. Philosophical Transactions of the Royal Society of London B 365:1299-1303.2000.
- Popper, A.N, Ramcharitar, J. U. & Campana, S.E., 2005. Why otoliths Insights from inner ear physiology and fisheries biology. Mar Fresh w Res, 56, 497–504.
- Ruck J.G. 1976. Studies on the development and osteology of some New Zealand in shore Fishes (PhD thesis). Wellington: Victoria University of Wellington.
- Schulzmirbach T, Reichenbacher B. Reconstruction of Oligocene and neogene freshwater fish faunas-an acutualistic study on cyprniiformotolith. Actapalaeontol Pol: 51(2):283-304. 2006.
- Secor, D.H. & Dean, J. M., 1989. Somatic growth effects on the otolith- fish size relationship in young pound- reared striped bass, Morone saxatilis. Can J. Fish. Aquat. Sci. 46,113-121.
- Volpedo, A.Echererria, D.D.2003. Ecomorphological patterns of the sagitta in fish on the continental shelf off Argentine, Fisheries Research, 60:551-560.
- Waessle, J.A., Lasta, C.A., & Favero, M., 2003. Otolith morphology and body size relationships for juvenile Sciaenidae in the Rio de la Plata estuary. Scientia Marina1 67, 233-240.
- West, C. J. & Larkin P. A., 1987. Evidence of size-selective mortality of juvenile sockeye salmon (Oncorhynchus nerka) in Babine Lake, British Columbia. Can. J. Fish. Aquat. Sci. 44, 712–721.
- Wyllie, E. T., 1987. Relationship of otolith length to total length in rockfishes from northern and central California. Fish. Bull. 85, 383–387.

This work is licensed under a Creative Commons Attribution 4.0 International License.





