How to Cite | Publication History | PlumX Article Matrix
Experimental Evaluation Of Albizia Lebbeck Benth. Stem Bark for Wound Healing Activity in Mice
Nilesh Gupta and U. K. Jain*
Bhopal Institute of Technology and Science, Pharmacy Bhojpur Road, Bangrasia, Bhopal - 462 045 India.
Corresponding Author E-mail:ukjain65@gmail.com
ABSTRACT: The present study was aimed to evaluate the wound healing activity of extract of bark part of Albizia lebbeck. It is well-known plant in Indian traditional medicines. On the basis of traditional use and literature references, this plant was selected for wound healing potential. The methanolic extract of bark parts of Albizia lebbeck was examined for wound healing activity in the form of ointment in three types of wound models on mice: the excision, the incision and dead space wound model. The extract ointments showed considerable response in all the above said wound models as comparable to those of a standard drug Betadine ointment in terms of wound contracting ability, wound closure time, tensile strength and dry granuloma weight. Histological analysis was also consistent with the proposal that Albizia lebbeck bark extract exhibits significant wound healing potential.
KEYWORDS: Albizia lebbeck; Wound healing activity; Betadine; Methanolic extract
Download this article as:| Copy the following to cite this article: Gupta N Jain U. K. Experimental Evaluation Of Albizia Lebbeck Benth. Stem Bark for Wound Healing Activity in Mice. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Gupta N Jain U. K. Experimental Evaluation Of Albizia Lebbeck Benth. Stem Bark for Wound Healing Activity in Mice. Biosci Biotech Res Asia 2010;7(2). Available from:http://www.biotech- asia.org/?p=9275 |
Introduction
The use of herbs and medicinal plant as the first medicines is a universal phenomenon. Every culture on the earth, through written or oral tradition, has relied on the vast variety of natural chemistrie’s found in plants for their therapeutic properties. Each drug extracted from the plants is substance with a particular therapeutic action1. The usage of herbal plants as traditional health remedies is the most popular for 80% of the world population in Asia, Latin America and Africa and is reported to have minimal side effects2.
Albizia lebbeck Benth. (Bengali name: Shirish, Koroi; Family: Leguminosae) is a deciduous tree with compound leaves, flat oblong fruits, round cream colored seeds, grows wild and planted in almost all districts of Bangladesh3. The plant is found throughout India, Bangladesh, tropical and subtropical Asia and Africa. Bark is pale with glabrous young shoot. The bark is bitter, cooling, alexiteric, anthelmintic, cures “vata”, diseases of the blood, leucoderma, itching, skin disease, excessive perspiration, inflammation, and good in rat bite. The bark is good for opthalmia. Barks are used in toothache and diseases of the gum. Decoction of the leaves and barks are protective against bronchial asthma and other allergic disorders. Barks and seeds are astringent and are given in piles and diarrhea4. Ethanolic extract of pods possesses antiprotozoal, hypoglycemic and anticancer properties. The methanolic extract of the pod was investigated for antifertility activity5-6. The plant extracts also evaluated in allergic rhinitis7, memory and learning of mice8, analgesic and anti–inflammatory9. All parts of the plant are recommended for the treatment of snake-bite10. It is reported to possess anxiolytic11, anticonvulsant12-13, antifertility14 and antidiarrhoeal activities15. Research on plants with medicinal properties and identification of the chemical components responsible for their activities have corroborated the traditional uses of ancient healing wisdom and lore and have proven the enduring healing potential of many plant medicines even in today’s hi-tech community.
Normal wound healing response begins the moment the tissue is injured. Wound healing is the process of repair that follows injury to the skin and other soft tissues. Following injury, an inflammatory response occurs and the cells below the dermis begin to increase collagen production. Later, the epithelial tissue is regenerated16. Impaired wound healing may cause severe health-related complications, such as infections and tissue necrosis. These aliments have spurred the search for wound healing agents derived from ethno medicinal sources17.
Current methods used to treat wounds include debridement, irrigation, antibiotics, tissue grafts, proteolytic enzymes, and corticosteroids, which possess major drawbacks and unwanted side effects. Therefore, the development of potent wound healer drugs with fewer side effects is necessary. Although Albizia lebbeck bark has traditionally been used in the treatment of many types of skin disease and inflammatory conditions in India., in villages people use plant extract to treat their wounds without any experimental knowledge. Literature search revealed that there is no experimental basis to prove the wound healing activity of Albizia lebbeck bark. Therefore, this study has been undertaken to assess the wound healing activity of methanolic extract of Albizia lebbeck bark in mice.
Material and Methods
Plant material
The stem bark of Albizia lebbeck was collected from mature trees and its botanical identification was confirmed from National Botanical Research Institute Lucknow. A voucher specimen NBRI/CIF/Re./08/2008/32 was deposited in the herbarium of NBRI Lucknow. The plant material was dried in shade, powdered and sieved through 40-mesh size and material was stored in well-closed container.
Extraction and preparation of formulation
Bark of Albizia lebbeck Benth was finely pulverized (100 g) and extracted in Soxhlet apparatus for 24 h with methanol then concentrated and dried under reduced pressure. The extract was weighed and the yield obtained (13.70% w/w). The semisolid mass (dark brown colour) was obtained and used as ingredient for 5% ointment preparation. About 5 g of semisolid extract was incorporated into the 100 g of simple ointment base B.P.. Simple ointment base was used as control group. Extract ointment was used ones daily to treat different groups of animals.
Animals
Healthy Albino mice of either sex (35–45 g) with no prior drug treatment were used for all the present in-vivo studies. The animals were fed on a commercial pellet diet (Hindustan Lever, Bangalore, India), and water ad libitum. The animals were acclimatized to laboratory hygienic conditions for 10 days before starting the experiment. Animal study was performed with due permission from institutional animal ethical committee.
Wound healing activity
Grouping of animals
Screening for wound healing activity was performed for incision, excision and dead space wound model. 54 animals of either sex weighed between 35 and 40 g were divided into three groups based on treatment type in each model consisting of six animals as follows: group I – simple ointment base; group II – 5% Betadine (Win-Medicare, India) ointment and group III –5% methanolic extract ointment of Albizia lebbeck. The hairs on the skin of white surface of animals were removed by using a suitable depilatory (Anne-French hair removing cream).
Excision wound model
Circular wounds of approximately 10mm diameter were inflicted on the cleared skin by cutting under ether anesthesia. The areas of the wounds were measured (sq. mm) immediately by using vernier calipers. This was taken as the initial wound area reading. Group-I animals served as control (negative control), which received ointment I.P. Group-II served as standard (positive control) to which Betadine (5% w/w in ointment I.P.) was applied topically. Group-III animals served as test, which were treated with the extract (5% w/w) in a similar manner. All the test samples were applied once daily. The wound area of each animal was measured on 1st, 4th, 7th, 10th, and 13th post wounding day (Table 1).The wound closure was measured at regular intervals of time to see the percentage of wound closure and epithelialization time that indicates the formation of new epithelial tissue to cover the wound. The number of days required for falling of the scar without any residual of the raw wound gave the period of epithelialization.
Table 1: Effect of Methanolic Extract Ointment of Albizia lebbeck Benth on Exsicion Wound Model.
|
S.N. |
Treat mnt |
Period of epithelizaion |
% wound contraction, post wounding day (Mean ± SD, n=6) |
% Un-healed Wound area mm2 , 13th post wounding day | |||
| 4th Day Mean ± SD | 7th Day Mean ± SD | 10th Day Mean ± SD | 13th Day Mean ± SD | 13th Day Mean ± SD | |||
| 1 | Control | 22nd days
|
26.55 ± 1.62 | 31.38 ± 1.04 | 62.49 ±0.61 | 73.8 ± 1.68
|
26.2 ± 1.68 |
| 2 | Standard | 11th days | 47.8 ± 1.83
|
73.28 ± 0.75
|
97.48 ± 0.63
|
99.6± 0.02**
|
.04 ± 0.02 |
| 3 | Test (Extract treated) | 13th days | 41.7 ± 0.73
|
55.34 ± 0.86
|
86.88± 0.75
|
97.6 ±0.68**
|
2.4 ± 0.68 |
P<0.05, **P<0.001 vs. control.
Incision wound model
All animals were anaesthetized before wound creation and paravertebral long incisions were made through the skin at the distance of about 1.5 cm from midline on the depilated back of mice. No local or systemic antimicrobials were used throughout the experiment. All groups were treated same as in excision model, the both edges kept together and stitched with black silk surgical thread (no. 000) and a curved needle (no. 11) was used for stitching. The continuous threads on wound edges were tightened for good closure of the wound. After stitching, wound was left undressed then simple ointment base, extract ointment and standard ointment were applied daily up to 7 days; when wounds were cured thoroughly the sutures were removed on the day 8th and tensile strength18, of cured wound skin was measured using tensiometer (Table 2).The skin breaking strength is expressed as the minimum weight (in grams) of water necessary to bring about the gapping of the wound.
Table 2: Effect of Methanolic Extract Ointment of Albizia lebbeck Benth on Insicion Wound Model.
| S.NO. | Treatment | No. of Animals | Tensile strength in gm/cm on 8th post wounding day (Mean ± SD, n=6) |
| 1 | Control | 6 | 169.62 ± 2.71 |
| 2 | Standard | 6 | 304.87± 1.25* |
| 3 | Test (Extract treated) | 6 | 254.87 ± 1.54* |
P<0.05 vs. control.
Dead space wound model
This model was used for the study of granuloma tissue. Animals were anaesthetized by ether anesthesia and wound was made by implantation of cotton pellets (10 mg), (2.0×0.5), one on side, in the lumber region on the dorsal surface in each animal. On the 8th post-wounding day, granuloma tissue formed on implanted cotton pellets was dissected out carefully. Granuloma tissue from one part was dried (600C) and weighted, while the other part of granuloma tissue19-20 was used for determination of tensile strength (Table 3).
Table 3: Effect of Methanolic Extract Ointment of Albizia lebbeck Benth on Dead Space Wound Model.
| S.NO. | Treatment | No. of Animals | Granulation Tissues dry-weight, mg/100gm
(Mean ± SD, n=6) |
Tensile strength in gm/cm on 8th post wounding day (Mean ± SD, n=6) |
| 1 | Control | 6 | 17.12 ± 0.85 | 159.12 ± 1.75 |
| 2 | Standard | 6 | 43.62± 0.47* | 317.37 ± 2.13* |
| 3 | Test (Extract treated) | 6 | 34.12 ± 0.85* | 284.37 ± 1.10* |
P<0.05 vs. control.
Histopathological Studies
Wound tissue specimens from control, test and standard groups were taken after complete healing of excision wound. After usual processing, 6-mm thick sections were cut and 10% of nautral formalin solution was used to fix the granulation tissues for 24 h and dehydrated with a sequence of ethanol- xylene series of solutions. The inflicted materials were embedded with paraffin at 40-60 0c. Microtome sections were taken at10m thicknesses. The processed sections were stained with haematoxylin eosin21-22 and observed under microscope.
Wound healing evaluation parameters
Wound contraction and epitheliazation time
An excision wound margin was traced after wound creation by using transparent paper and area measured by vernier calipers. Wound contraction was measured in each 3 days interval, until complete wound healing and expressed in percentage of healed wound area. The epitheliazation time23 was measured from initial day.
Measurement of tensile strength
Tensile strength is the resistance to breaking under tension. It indicates how much the repaired tissue resists to breaking under tension and may indicate in part the quality of repaired tissue. Sutures were removed on the day 8 after wound creation and the tensile strength was measured. For this purpose, the newly formed tissue including scar was excised and tensile strength was measured with the help of tensiometer, which is based on method of Kuwano24. In this method wound breaking strength was measured as the weight of water at the time of wound breaking per area of the specimen.
Dry granulation weight
From the collected tissue 8th day skin sample of dead space wound ( granulomas ) was excised from cotton pellets and granuloma mass was dried and weighed, while the other part of granuloma tissue was used for determination of tensile strength.
Histopathological studies
The healing tissues obtained on the 13th day from all three groups of animals of the excision wound model were processed for histological study. Sections were qualitatively assessed under the light microscope and observed in respect of fibroblast proliferation, collagen formation, epithelialization and blood vessels.
Statistical analysis
Treated group was compared with the control group. The results were analyzed statistically using Student’s t-test to identify the differences between the treated and control. The data were considered significant at p<0.05.
Results & Discussions
The percentage wound contraction25 was determined using the following formula:

Wound area was measured by tracing the wound margin using vernier calipers in each 3 days interval and healed area was calculated by subtracting from the original wound area. On day 7, the wound contraction of standard and test were found to be significant (P<0.05) in comparison to control group. On day 10, wound was completely healed for standard group while for test group it was also almost at complete healing stage. On day 13, test group healed 97.6% and control group showed 73.8% healing. It was also observed that epitheliazation period of test and standard group were less in comparison to control group (Table 1). The time required for complete epitheliazation of the excision wound is an important parameter to assess the wound healing process.
The studies on excision wound healing model reveal that all the three groups showed day to day decrease in wound area. However, on 13th post wounding day, control animals group-I showed 26.2 ± 1.68 of unhealed wound area whereas group-II standard group animals, showed that of .04 ± 0.02 and the test group-III exhibited that of 2.4 ± 0.68 wound area. When compared with the control, the activity of extract was found to be highly significant (P<0.05).
The promotion of wound healing activity is also well gazed by its tensile strength of the incision wound. Generally wound-healing agents have the properties to enhance the deposition of collagen content, which provides strength to the tissues and forms cross-linkages between collagen fibers. The tensile strength of the test group was found to be (254.87 ± 1.54) which was higher than that of control group (169.62 ± 2.71) of animals and slightly lesser than that of standard group (304.87± 1.25) of animals on 8th post wounding day.
The effect of topical administration of the test group and control treated group on dead space wound model was assessed by increase in the weight of granulation tissue and increase tensile strength. The data is depicted in table-3. This indicates enhanced collagen maturation by increased cross-linking of collagen fibers. The increased weight of the granulation tissue also indicated the presence of higher protein content. Among these treated animals the response was shown to be the best in test animals.
Histology of the wound tissue of the control animals showed the presence of acute inflammatory cells, fibroblastic connective tissue and very little number of blood vessels (Fig. 1A). The lesser epithelialization and lesser collagen formation indicated incomplete healing of the wound in control animals. Where as, in the sections of standard treated group animals (Fig. 1B) increased collagen deposition was observed. The sections of the granuloma tissue of the animals treated with methanolic extract showed moderate epithelialization, fibrosis, collagen formation and increased number of blood vessels (Fig. 1C)
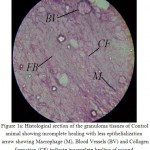 |
Figure 1A. Histological section of the granuloma tissues of Control animal showing incomplete healing with less epithelialization arrow showing Macrophage (M), Blood Vessels (BV) and Collagen formation (CF) indicate incomplete healing of wound.
|
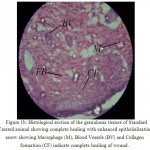 |
Figure 1B. Histological section of the granuloma tissues of Standard Treated animal showing complete healing with enhanced epithelialization arrow showing Macrophage (M), Blood Vessels (BV) and Collagen formation (CF) indicate complete healing of wound.
|
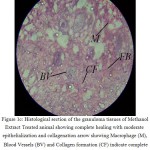 |
Figure 1C. Histological section of the granuloma tissues of Methanol Extract Treated animal showing complete healing with moderate epithelialization and collagenation arrow showing Macrophage (M), Blood Vessels (BV) and Collagen formation (CF) indicate complete healing of wound.
|
Conclusions
The study has showed that the methanolic extract ointment of Albizia lebbeck effectively stimulates wound contraction; increase tensile strength of incision and dead space wounds as compared to control group. This was demonstrated by a significant increase in the rate of wound contraction and by enhanced epithelization. Significant increase (P<0.05) in tensile strength, collagen levels and weight of granulomas were observed, which were further supported by histopathological studies and gain in granuloma breaking strength. This indicated improved collagen maturation by increased cross-linking while an increase in dry granuloma weight indicated accumulation of higher protein content. These finding could justify the pronounced wound healing activity of this plant.
Acknowledgement
The authors are thankful to Bhopal Institute of Technology & Science-Pharmacy, Bhojpur Road, Bangrasia, Bhopal for providing facilities to carry out the research work.
References
- Serrentino, J., How Natural Remedies Work. Harley and Marks Publishers, Point Robert, WA, 20-22 (1991).
- Doughari, J. H., “Antibacterial activity of Tamarindus indica Linn.” Trop. J. Pharm. Res., 5: 597-603 (2006).
- Ghani A., In: Medicinal Plants of Bangladesh with Chemical Constituents and Uses, 2nd edn. Asiatic Society of Bangladesh, 81 (2003).
- Kirtikar, K. R. and Basu, B. D., In: Indian Medicinal Plants, (Singh B and Singh MP eds). India, Part II, 937 (1980).
- Gupta, R. S., Kachhawa, J. B. Chaudhary, R., “Antifertility effects of methanolic pod extract of Albizia lebbeck Benth. in male rats.” Asian J. Androl., 6(2): 155-159 (2004).
- Gupta, R. S., Chaudhary, R., Yadav, R. K., Verma, S. K. and Dobhal, M. P., “Effect of Saponins of Albizia lebbeck Benth. bark on the reproductive system of male albino rats.” J. Ethnopharmacol., 96(1-2): 31-36 (2005).
- Pratibha, N., Saxena, V. S., Amit, A., D’Souza, P., Bagchi, M. and Bagchi, D., “Anti-inflammatory activities of Aller-7, a novel polyherbal formulation for allergic rhinitis.” Int. J. Tissue. React., 26(1-2): 43-51 (2004).
- Chintawar, S. D., Somani, R. S., Kasture, V. S. and Kasture, S. B., “Nootropic activity of Albizia lebbeck in mice.” J. Ethnopharmacol., 81(3): 299-305 (2002).
- Achinto, S. and Munirudin, A., “The Analgestic and anti-inflammatory activities of the extract of Albizia lebbeck in animal model.” Pak. J. Pharm. Sci., 22: 74-77 (2009).
- Kirtikar, K. R. and Basu, B. D. Indian Medicinal Plants, Vol. II, 2nd edn. International
- Book Distributor, Dehra Dun, India, 936-938 (1999).
- Une, H. D., Sarveiya, V. P., Pal, S. C., Kasture, V. S. and Kasture, S. B., “Nootropic and anxiolytic activity of saponins of Albizia lebbeck leaves.” Pharmacology Biochemistry and Behavior, 69: 439-444 (2001).
- Kasture, V. S., Chopade, C. T. and Deshmukh, V. K., “Anticonvulsive activity of Albizia lebbeck, Hibiscus rosa sinesis and Butea monosperma in experimental animals.” Journal of Ethnopharmacology, 71: 65-75 (2000).
- Kasture, V. S., Kasture, S. B. and Pal, S. C., “Anticonvulsant activity of Albizia lebbeck leaves.” Indian J of Exp Biol., 34: 78-80 (1996).
- Gupta, R. S., Kachhawa, J. B., and Chaudhary, R., “Antifertility effects of methanol pod extract of Albizia lebbeck (L.) Benth in male rats.” Asian J Andro., 6: 155-159 (2004).
- Besra, S. E., Gomes, A., Chaudhury, L., Vedasiromoni, J. R. and Ganguly, D. K., “Antidiarrhoeal activity of seed extract of Albizia lebbeck Benth.” Phytotherapy Research, 16: 529-533 (2002).
- Shivanand, N., Poorna, N., Steve, S., Vidyasagar, B. and Andrew, A., “Evaluation of wound healing activity of Allamanda cathartica. L.and Laurus nobilis. L.” BMC.Compl Altern. Medicine, 6: 12-16 (2006).
- Letts, G. M., “In vivo wound healing activity of oleanolic acid derived from the acid hydrolysis of Anredera diffusa.” Journal of Nat. Product, 69: 978-978 (2006).
- Hemalata, S., Subramanian, N., Ravichandran, V. and Chinnaswamy, K., “Wound healing activity of Indigofera ennaphylla Linn.” Indian Journal of Pharmaceutical Sciences, 63: 331–333 (2001).
- Shirwaikar, A., Jahagirdar, S. and Udupa, A. L., “Wound healing activity of Desmodium triquetrum leaves.” Indian Journal of Pharmaceutical Sciences, 65: 461–464 (2003).
- Patil, M. B., Jalalpure, J. S. and Ashraf, A., “Preliminary phytochemical investigation and wound healing activity of the leaves of Argemone maxicana Linn.” Indian Drugs, 36: 288–293 (2001).
- Varshney, A. C., Sharma, D. N., Mohinder, S., Sharma, S. K. and J. M. Nigam., “Therapeutic value of Bovine Saliva in wound healing a histo-marphological study.” Ind. J. Exp. Biol., 35: 535- 537 (1997).
- Manus, M. C., and Mowry, R. W., Staining Methods, Histologic and Histochemical. Harper & Row/Evanston, New york/London, (1965).
- Rashed, A. N., Afifi, F. U. and Disi, A. M. “Simple evaluation of wound healing activity of a crude extract of Portuloca oleracea Linn. (growing in Jordan) in Mus musculus JVI-1.” Journal of Ethanopharmacology, 88:131–136 (2003).
- Kuwano, H., Yano, K., Ohano, S., Ikebe, M., Kitampura, K., Toh, Y., Mori, M. and Sugimachi, K., “Dipyridamole inhibits early wound healing in rat skin incisions.” Journal of Surgical Research, 56: 267–270 (1994).
- Srivastava, P. and Durgaprasad, S., “Burn wound healing properties of Cocos nucifera –An appraisal.” Indian J Pharmacol., 40: 144-6 (2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.





