How to Cite | Publication History | PlumX Article Matrix
V. C. Khilare* and S. S. Chavan
Botany Research Centre, Vasantrao Naik Mahavidyalaya, CIDCO, Aurangabad - 431 003 India.
Corresponding Author E-mail: vikramkhilare@gmail.com
ABSTRACT: Fungicide carbendazim is recommended to manage anthracnose of grapes in orchards which is important disease in India. The wild sensitive isolate GA-1 was studied both in vitro and in vivo on grapes. Culturing wild type isolate continuously for five successive passages on carbendazim individually increased resistance significantly. However, reduced resistance was observed when pathogen was cultured alternately or in mixture with different fungicides of amide and conazole groups. Similar type of results was obtained on the grape berries. Use of difenoconazole and myclobutanil alternately and difenoconazole, myclobutanil and propiconazole in mixture appeared to be most useful to break the development of carbendazim resistance in pathogen.
KEYWORDS: Fungicide resistance; Carbendazim; Anthracnose of grapes; Gloeosporium ampelophagum
Download this article as:| Copy the following to cite this article: Khilare V. C, Chavan S. S. Effect of Passage on the Development of Carbendazim Resistance in Gloeosporium ampelophagum Causing Anthracnose of Grapes. Biosci Biotech Res Asia 2011;8(2) |
| Copy the following to cite this URL: Khilare V. C, Chavan S. S. Effect of Passage on the Development of Carbendazim Resistance in Gloeosporium ampelophagum Causing Anthracnose of Grapes. Biosci Biotech Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9309/ |
Introduction
The grape (Vitis vinifera) is one of the most economically important fruit crops in the world1. Anthracnose is one of the most damaging diseases of grape and is caused by Gloeosporium ampelophagum (Pass.) Sacc. and responsible for yield losses in commercial grape production. In wet humid regions the disease incidence and severity on various cultivars of grape can be very serious2. Infection may occur on all succulent plant material but is most common on fruit and shoots. Lesions on berries are initially small, circular and reddish in color. Acervuli are also produced in these lesions. Leaf spots are often numerous and resemble those on fruit. The center often drops out leaving a shot hole appearance. Young leaves are more susceptible than older leaves and are malformed when veins become infected. Fungicides have been extensively used to control anthracnose of grape, but cause environmental pollution and leave residues in the agricultural soil and on products. Chemical usage has been effective, although resistance to these fungicides is developing. The development of carbendazim resistance against G. ampelophagum in Maharashtra3 and other States of India was studied by many workers4 -10.
Materials and Methods
Sensitivity of pathogen
The infected samples of grapes were collected from different districts known as ‘grape belt’ of Maharashtra like Ahmednagar, Nashik, Pune and Solapur during 2009 & 2010 crop seasons. Isolation of pathogen was done by inoculating the samples on Czapek-Dox agar medium. The cultures were further purified and maintained on same medium at 27± 1° C. A total of 37 isolates were purified and tested against carbendazim fungicide by ‘Poisoned Food Technique’11 to check their sensitivity. Czapek-Dox agar medium (2X) was prepared and it was then sterilized and 10 ml of this was properly mixed with 10 ml of fungicide (2X a.i. concentrations) selected for study in sterile Perti plates. A series of concentrations was prepared; the fungicide was thoroughly mixed with medium and allowed to solidify. A 4 mm disc of the fresh grown G. ampelophagum isolates was transferred aseptically at the centre of Petri plate. On the basis of minimum inhibitory concentration (MIC) the resistant and highly resistant population was calculated by multiplying four times to the sensitive baseline dose as per guidelines given by fungicide resistance action committee (FRAC). The data of radial growth was analyzed for MIC and essential dose (ED 50) by using following equation12.
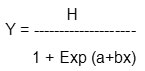
(Where, Y = radial growth as percentage of control, H= upper limit of curve, Exp= logarithmic exponent, a= regression constant, b= regression coefficient and x= measured points).
Study of Passage
In order to study the effect of passage in vitro wild sensitive isolate GA-9 in each passage was cultured on agar plates containing sub-lethal dose of carbendazim (0.3 μg/ml). The plates without fungicide served as control. A 4 mm diameter disc of freshly grown culture taken from the culture of previous passage of the same isolate was placed at the centre of each plate. In each passage linear growth was measured after eight days. Percentage increase of growth of the isolate from passage to passage was considered as increase in carbendazim resistance or vice-versa. The development of resistance thus was studied up to 5th passage. Alternate passage carbendazim with triadimefon, metalaxyl, difenoconazole, myclobutanil, propiconazole and mixed passage with the same fungicide were also carried out.
Passage studies were also carried out on the grape berries (Vitis vinifera L. var. Thomson seedless). The grape berried were inoculated with spore suspension taken from the culture of previous passage of the same isolate was inoculated on grape berries. The concentration of carbendazim and wild sensitive isolate GA-9 was kept same as used in vitro studies. At each passage percent disease index (PDI) was calculated. PDI increased from passage to passage considered as increase in the carbendazim resistance. The development of resistance was studied up to 5th passage both in alternate and in mixture of different fungicides.
Results and Discussion
Results in Table 1 indicate that in vitro individually culturing of the pathogen in carbendazim increased growth significantly up to 5th passage. However, alternate culturing of pathogen on difenoconazole and myclobutanil reduced growth significantly. This reduction was more prominent with myclobutanil than other fungicides. Interestingly there was also significant reduction in the growth of pathogen when cultured on the carbendazim in combination with difenoconazole and myclobutanil on grape berries (Table 2). In vivo results are given in Table 3 and 4. It was seen that again treatment of carbendazim to grape berries for five successive passages increased PDI on grapes. However, treatment of carbendazim alternately with triadimefon, metalaxyl, difenoconazole, myclobutanil and propiconazole reduced PDI significantly. Use of carbendazim with difenoconazole, myclobutanil and propiconazole were most useful in controlling grape anthracnose. There are some evidences for increase of resistance due to continuous exposure of pathogen to the fungicides12, 13. Alternate or mix application of fungicide must have different mode of action14 and in the present investigation there might have less chances to mutate or to adopt resistance in G. ampelophagum due to use of other group of fungicides. These results also agree with the earlier work in case of Septoria nodorum against carbendazim12. Similar results were obtained by author in Penicillium digitatum against thiophanate-methyl and in Alternaria alternata against aureofungin15,16 causing fruit rot of grapes.
Table 1: Effect of continuous exposure to carbendazim and to carbendazim alternately with other different fungicide on growth of Gloeosporium ampelophagum on agar medium during five successive passages.
| Sr.
No. |
Fungicides (0.3μg/ml) |
Passage number | ||||
| I | II | III | IV | V | ||
| 1 | Carbendazim continuous
|
18.57 | 24.35 | 48.57 | 70.00 | 73.61 |
| 2 | Carbendazim alters triadimefon
|
18.57 | 94.40 | 35.71 | 87.14 | 70.83 |
| 3 | Carbendazim alters metalaxyl
|
18.57 | 95.83 | 32.85 | 88.57 | 34.72 |
| 4 | Carbendazim alters difenoconazole
|
18.57 | 51.38 | 34.28 | 57.14 | 09.16 |
| 5 | Carbendazim alters myclobutanil
|
18.57 | 97.20 | 35.71 | 81.42 | 11.08 |
| 6 | Carbendazim alters propiconazole
|
18.57 | 48.61 | 72.85 | 80.00 | 61.11 |
Table 2: Effect of continuous exposure to carbendazim and to carbendazim alternately with other fungicides on growth of Gloeosporium ampelophagum on grape berries during five successive passages.
| Sr.
No |
Fungicides (0.3μg/ml) |
Passage number | ||||
| I | II | III | IV | V | ||
| 1 | Carbendazim continuous
|
52.22 | 59.78 | 65.12 | 70.14 | 75.43 |
| 2 | Carbendazim alters triadimefon
|
52.22 | 88.28 | 44.30 | 75.18 | 66.40 |
| 3 | Carbendazim alters metalaxyl
|
52.22 | 60.12 | 47.14 | 76.44 | 32.18 |
| 4 | Carbendazim alters difenoconazole
|
52.22 | 82.20 | 56.16 | 78.15 | 21.85 |
| 5 | Carbendazim alters myclobutanil
|
52.22 | 94.12 | 62.18 | 81.00 | 15.22 |
| 6 | Carbendazim alters propiconazole
|
52.22 | 62.46 | 78.15 | 84.67 | 58.10 |
Table 3: Effect of continuous exposure to carbendazim and to carbendazim mixed with other different fungicide on growth of Gloeosporium ampelophagum on agar medium during five successive passages.
| Sr.
No. |
Fungicides (0.3μg/ml) |
Passage number | ||||
| I | II | III | IV | V | ||
| 1 | Carbendazim continuous
|
21.25 | 33.33 | 38.57 | 39.18 | 40.00 |
| 2 | Carbendazim mixed triadimefon
|
34.61 | 48.33 | 39.74 | 32.50 | 32.89 |
| 3 | Carbendazim mixed metalaxyl
|
37.17 | 50.00 | 44.44 | 35.00 | 36.52 |
| 4 | Carbendazim mixed difenoconazole
|
34.28 | 21.93 | 16.11 | 07.17 | 05.52 |
| 5 | Carbendazim mixed myclobutanil
|
40.00 | 38.70 | 37.50 | 35.89 | 35.52 |
| 6 | Carbendazim mixed propiconazole
|
22.85 | 27.41 | 22.36 | 24.35 | 20.68 |
Table 4: Effect of continuous exposure to carbendazim and to carbendazim mixed with other different fungicide on growth of Gloeosporium ampelophagum on grape berries during five successive passages.
| Sr.
No |
Fungicides (0.3μg/ml) |
Passage number | ||||
| I | II | III | IV | V | ||
| 1 | Carbendazim continuous
|
23.78 | 43.69 | 57.42 | 66.90 | 72.14 |
| 2 | Carbendazim mixed triadimefon
|
38.15 | 49.77 | 43.24 | 40.78 | 40.00 |
| 3 | Carbendazim mixed metalaxyl
|
42.66 | 56.90 | 46.11 | 41.89 | 42.00 |
| 4 | Carbendazim mixed difenoconazole
|
36.08 | 14.15 | 13.80 | 09.68 | 09.32 |
| 5 | Carbendazim mixed myclobutanil
|
41.90 | 39.00 | 37.08 | 34.88 | 32.18 |
| 6 | Carbendazim mixed propiconazole
|
27.10 | 34.11 | 27.00 | 30.83 | 21.66 |
Acknowledgement
Authors are grateful to the University Grants Commission, New Delhi for providing financial support in the form of major research project to carry out these investigations. Thanks are also due to the Principal, Vasantrao Naik College for providing the facilities to complete this work.
References
- Roger, C.P. and Goheen, A.C.: Compendium of Grape Diseases. 4th APS Press, St. Paul, Minnesota, USA, (1998)
- Kummuang, N., Smith, B.J., Diehl, S.V. and Graves, J. Pl. Dis. 80: 238 (1996)
- Deokate, A.S., Khilare, V.C. and Gangawane, L.V.: J. Plant Prot. 30: 69 (2002)
- Reddy, M.S., Rama, P. and Appa Rao, A.: Indian Phytopath. 33: 450 (1980)
- Reddy, M.S., Rama, P. and Appa Rao, A.: Proc Indian Acad Sci (Plant Sci) 90: 535 (1981)
- Kumar, S. and Thind, T.S.: Plant Dis. Res. 7: 103 (1992)
- Thind, T.S., Mohan, C., Kumar, S. and Azmi, D.P.: Indian J. Mycol. Pl. Patho. 24:46 (1994)
- Chander, M. and Thind, T. S.: J. Mycol. Plant Pathol. 24: 25 (1995)
- Mohan, C. and Thind, T.S.: J. Mycol. Pl. Pathol. 25: 25 (1995)
- Nene, Y. L. and ThapliyalN., Fungicides in Plant Disease Control. Oxford and IBH Publishing Co. Pvt. Ltd. New Delhi 691 (1993)
- Molnar, A. Hornok, L. and Pest, M.: Experimental Mycol. 9: 326 (1985)
- Horstein, J.A.H.M.: Acquired resistance to systemic fungicides of Septoria nodorum and Cercosporella herpotrichoides in cereals. Dissertation, Agricultural Univ. Wageningen, Netherlands, 107 (1979)
- Gangawane, L.V. and Shah, A.: Ind. Phytopath. 41: 638 (1988)
- Griffin, M.J., Plant Pathology Notes No. 38. Fungicide resistance. ADAS South Western Region, UK. (1981)
- Khilare, V.C. and Gangawane, L.V.: Ind. Bot.Soc. 77: 237 (1998)
- Kadam, K.S., Khilare, V.C. and Gangawane, L.V., Frontiers in Fungal Biotechnology and Plant Pathogen Relations, Proc. Confr. 16-18, Jan. 1999, Allied Publishers Ltd. New Delhi, 259 (1999)

This work is licensed under a Creative Commons Attribution 4.0 International License.





