Manuscript accepted on : September 17, 2010
Published online on: 28-12-2010
Production of Alpha Amylase Using Bacillus Subtilis Ncim No. 2724 under Solid State Fermentation
B. Manuel Choragudi1*, B. Ranjani Choragudi1, S. Felice Choragudi2 and V. Saradhi Settaluri2
1Sujay Bio-Tech Pvt. Ltd., Plot No.19A, Phase - III, 3rd Road, Jawahar Autonagar, Vijayawada India.
2Koneru Lakshmaiah College of Engineering, Green Fields, Vaddeswaram India.
Corresponding Author E-mail: ben_cmanuel@rediffmail.com
ABSTRACT: Production of alkaline amylase by Bacillus sp. under solid state fermentation (SSF) was optimized. The effect of wheat bran and rice bran were examined. The appropriate Incubation time, Temperature, pH, Inoculum size, Moisture content, Carbon source, Nitrogen source, Substrate concentration, Surfactant and Mutagenic effects were determined. Maximum yield of enzyme was achieved by employing wheat bran as substrate ((94±2) U/g) at 72hrs with 1:1.5 moisture content and pH-8. Inoculum size was found to be 30% for wheat bran. The increased growth of bacterium of mutagenic activity is seen with acrylamide solution.
KEYWORDS: Enzymes; alfa-amylase; wheat bran; rice bran
Download this article as:| Copy the following to cite this article: Choragudi B. M, Choragudi B. R, Choragudi S. F, Settaluri V. S. Production of Alpha Amylase Using Bacillus Subtilis Ncim No. 2724 under Solid State Fermentation. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Choragudi B. M, Choragudi B. R, Choragudi S. F, Settaluri V. S. Production of Alpha Amylase Using Bacillus Subtilis Ncim No. 2724 under Solid State Fermentation. Biosci Biotech Res Asia 2010;7(2).Available from:https://www.biotech-asia.org/?p=9396 |
Introduction
Enzymes are proteins that catalyze the chemical reactions. In these reactions, the molecules at the beginning of the process are called substrates and the enzyme converts them into different molecules, the products. Almost all process in the cell need enzymes are extremely selective for their substrates and speed up only a few reactions from among many possibilities, the set of enzymes made in a cell determines which metabolic pathways occur in that cell.
The use of industrial enzymes has now extended to almost all industries handling organic compounds. Enzymes are used variably for example, as ingredients in detergents, reagents for analysis of drugs or blood components, food use or food additives, fiber processing or pulp processing in the paper industry and environmental treatment.
Amylases are starch-degrading enzymes, which primarily breaks down starches in food so that they can be used by the body. They are widely distributed in microbial, plant and animal kingdoms. They degrade starch and related polymers to yield products characteristic of individual amylolytic enzymes.
Amylases constitute a class of industrial enzymes having approximately 25% of the enzyme market. It is desirable that α-amylases should be active at high temperatures of gelatinization (100-110°C) and liquefaction (80-90°C) to economize processes.
Therefore there has been a need for more thermophilic and thermostable α-amylases. With the availability of thermostable enzymes, a number of new possibilities for industrial processes have emerged. While the most widely used thermostable enzymes are the amylases in the starch industry, a number of other applications are in various stages of development. Thermostable enzymes isolated from thermophilic organisms have found a number of commercial applications because of their overall inherent stability.
The spectrum of amylase application has widened in many other fields, such as clinical, medical, and analytical chemistries, as well as their wide spread application in starch sacccharification and in the textile, food, fermentation, paper, brewing and distilling industries.
Solid – State Fermentation
SSF is generally defined as the growth of microorganisms on moist solid substrates with negligible free water. The solid substrate may provide only support or both support and nutrition. SSF constitutes an interesting alternative since the metabolites so produced are concentrated and purification procedures are less costly. SSF is preferred to SmF because of simple technique, low capital investment, lower levels of catabolite repression and end- product inhibition, low waste water output, better product recovery, and high quality production. Among the different substrates used for SSF, wheat bran has been reported to produce promising results. Other substrates such as sunflower meal,
rice husk, cottonseed meal, soybean meal, and pearl millet and rice bran have been tried for SSF. SSF technique is generally confined to the processes involving fungi.
However, successful bacterial growth in SSF is known in many natural fermentations.
Research on the selection of suitable substrates for SSF has mainly been centered on agro industries. The utilization of these agro industrial wastes, on one hand, provides alternative substrates and, on the other, helps in solving pollution problems, which otherwise may cause their disposal.
Materails and Methods
Microorganism
The organism used in the present study is Bacillus subtilis NCIM 2724, purchased from NCL – Pune. The culture was routinely maintained in Nutrient Agar medium.Peptone (10gm/lt), Beef Extract powder (10gm/lt), NaCl(5gm/lt), Agar (20gm/lt). Fresh slants are prepared every fortnight to maintain the culture.
The inoculation was done by adding 2% of culture broth grown for 24 hrs from slants.
Fermentation conditions
Initial enzyme production was checked individually using wheat bran and rice bran. Commercial wheat bran (10gms) and Rice Bran (10gms) were finely mixed with 10ml
of distilled water in a 250ml Erlenmeyer flask and sterilized at 15lb/in2 for 20min.
After cooling, the flasks were inoculated with 10% inoculum size. The contents were mixed thoroughly, incubated at 37°C for 24 hrs, enzyme is extracted using PBS and assayed for enzyme activity.
Further optimization of process parameters was studied using wheat bran and rice bran as substrates for solid-state fermentation.
Development of the inoculum, enzyme production and extraction
For the development of inoculum, culture was transferred from stock to 100-mL nutrient broth (Starch 2gm, Beef extract 1gm, Peptone 1gm, NaCl 0.5gm, Distilled water 100ml) and the inoculated flasks were incubated overnight at (35±2) °C and 150 rpm.
Cells were harvested from the broth and their absorbance was checked at 660 nm. Production media contained 10 g of solid substrate and 10 mL of distilled water 250-mL Erlenmeyer flasks and were inoculated with the above inoculum. Inoculated production media were incubated under static conditions at (35±2)0C and enzyme production was checked after 24 hrs. Enzyme was extracted in 50 ml of 0.1 M phosphate buffer (pH=7) on a rotary shaker at 250 rpm for 30 min. The content was filtered through muslin cloth, filtrate was centrifuged at 8325 × g for 10 min and clear brown supernatant was used as the enzyme source.
Extraction of Enzyme
After 24 hrs of incubation, the brans were mixed with 15ml distilled water
(1:1.5 moisture content) and homogenized by centrifugation at 10,000 rpm for 15min. This yields the supernatant which is used as a crude enzyme source.
It can further be purified by partial purification methods like precipitation by Ammonium Sulphate and Acetone.
Analytical Methods
Partial Purification
Ammonium Sulphate Precipitation
To 1ml of crude enzyme extract add 1ml of Tris-HCl Buffer and 2ml of 50% Ammonium Sulphate and centrifuge at 5000 rpm for 10min.
To the pellet add 1ml of Tris-HCl Buffer and 2ml of 60% Ammonium Sulphate and centrifuge at 5000rpm for 10min.
To get the highest purity of enzyme the concentration of Ammonium Sulphate is varied from 50% to 80% and repeated.
The final pellet is resuspended in 1ml Tris – HCl Buffer and used as enzyme source.
Acetone Precipitation
Cool the required volume of acetone to -20°C.
Place protein sample in acetone-compatible tube.
Add four times the sample volume of cold (-20°C) acetone to the tube.
Vortex tube and incubate for 60 minutes at -20°C.
Centrifuge 10 minutes at 13,000-15,000 x g.
Decant and properly dispose of the supernatant, being careful to not dislodge the protein pellet.
Allow the acetone to evaporate from the uncapped tube at room temperature for 30 minutes. Do not over-dry pellet, or it may not dissolve properly.
Add buffer appropriate for the downstream process and vortex thoroughly to dissolve protein pellet.
The solution thus obtained is used as enzyme source for carrying out enzyme assay.
Enzyme Assay
α -Amylase activity was determined by incubating a mixture of 0.5 ml of aliquote of each enzyme source and 1 % soluble starch dissolved in 0.1 M phosphate buffer, pH=7,
at 55 °C for 15 min. The reaction was stopped by adding 1 ml of 3, 5-dinitrosalicylic acid, and then followed by boiling for 10 min. The final volume was made up to 12 ml with distilled water and the reducing sugar released was measured at 540 nm.
One unit (U) of α-amylase activity was defined as the amount of enzyme that releases
1 µmol of reducing sugar per minute, under assay conditions and expressed as U/g of dry substrate.
Enzyme activity calculation:
Units/gm = micromoles maltose released
gm enzyme in reaction mixture X 15 min
Parameters studied on Bacillus subtilis for Alpha amylase production
Effect of Inoculum size
Inoculum size was varied as 10, 20, 30 and 40 % (volume per mass) of inoculum and enzyme production was checked using wheat bran and rice bran as substrate and distilled water as moistening agent.
Effect of Moisture content
Substrate : moisture ratio was maintained as 1:1, 1:1.5, 1:2, 1:2.5, 1:3, and 1:3.5 ratios for 10% inoculum size and enzyme production is checked using wheat bran and rice bran.
Effect of Production time
Enzyme production is observed after 24hrs, 48hrs, and 72hrs using wheat bran and rice bran as substrates with 10% inoculum size.
Effect of additional nutrients
Carbon sources (0.04 g/g dry substrate) such as glucose, soluble starch, maltose and sucrose, and nitrogen sources (0.02 g/g dry substrate) as casein, yeast extract, peptone and thiourea were supplemented as individual components to the production media to check their effect on enzyme production.
Effect of temperature on enzyme activity
To determine temperature activity profile for α-amylase, enzyme assay was carried out at 35°C, 55°C, 60°C, 65°C and 75 °C.
Effect of pH on enzyme activity
For determination of suitable pH range for enzyme activity, assay was carried out by varying pH of enzyme assay buffer (phosphate buffer 0.1 M) was varied as 5, 6, 7, 8, 9 and 10.
Effect of substrate concentration on enzyme activity
Enzyme assay was carried out at different substrate concentrations 1%, 2%, 3%, 4%, 5% (starch dissolved in phosphate buffer 0.1M).
Effect of incubation time on enzyme activity
Enzyme assay was carried out at different incubation time periods 5, 10, 15, 20, 30 min i.e.; by varying the enzyme-substrate reaction time.
Effect of mutagenesis on enzyme production
Bacillus subtilis was mutated using potent mutants Ethidium Bromide(5mg/ml) in concentrations 10ml/ml of inoculum and 20ml/ml of inoculum, exposed to UV light for 10 min and 20min respectively and also using Acrylamide(5mg/ml) in concentrations 10ml/ml of inoculum and 20ml/ml of inoculum and checked for enzyme activity.
Results And Discussions
Bacillus species are considered to be the most important sources of α-amylase and have been used for enzyme production using SSF.Enzyme production was tested by varying different cultural parameters and the activity of amylase was tested by varying different parameters.Obtained results are as follows.
Effect Of Substrateon Enzyme Production
Wheat bran and rice bran were evaluated for a-amylase production by solid-state fermentation. Among the two substrates tested, highest enzyme production was observed with wheat bran ((94±2) U/g).
Maximum enzyme production observed after 72 h, which decreased with further incubation for both substrates. The decrease in enzyme production evident in the graphs shown (Fig 2 & 3) below may be due to the depletion of medium as the microorganisms consume the nutrients in it.
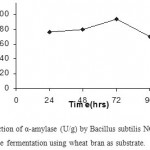 |
Figure 1: Production of α-amylase (U/g) by Bacillus subtilis NCIM 2724 under solid-state fermentation using wheat bran as substrate.
|
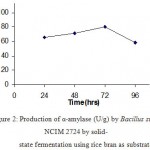 |
Fig.2. Production of α-amylase (U/g) by Bacillus subtilis NCIM 2724 by solid-state fermentation using rice bran as substrate.
|
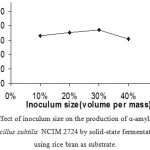
Effect Of Inoculum On Enzyme Production
Varying inoculum size of bacterial cells during the fermentation, 30 % (volume per mass) inoculum was found to be as optimum for the enzyme production. (Fig 4 &5) Increase in inoculum size was found to adversely affect the enzyme production.
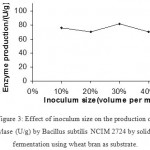 |
Fig.3. Effect of inoculum size on the production of α-amylase (U/g) by Bacillus subtilis NCIM 2724 by solid-state fermentation using wheat bran as substrate.
|
|
Figure 4: Effect of inoculum size on the production of α-amylase (U/g) by Bacillus subtilis NCIM 2724 by solid-state fermentation using rice bran as substrate.
|
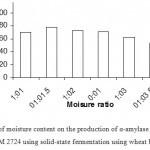 |
Figure 5: Effect of moisture content on the production of α-amylase (U/g) by Bacillus subtilis NCIM 2724 using solid-state fermentation using wheat bran as substrate.
|
Effect Of Moisture Content On Enzyme ProductioN
As the moisture content of the medium changes during fermentation as a result of evaporation and metabolic activities, adjusting the optimum moisture level of substrate during SSF is therefore most important. During solid-state fermentation, higher moisture level decreases porosity, changes wheat bran particle structure, promotes development of stickiness and lowers oxygen transfer, whereas lower moisture content causes reduction in the solubility of nutrients of the solid substrate, lower degree of swelling and higher water tension. In the present study, high enzyme titer was obtained when the substrate: moisture ratio was maintained as 1:1.5 in comparison with that of low or high moisture levels. Maximum a-amylase enzyme production by thermophilic B. coagulans has been reported using a high level of moisture at 1:2.5 ratio of substrate: moisture content.
(Fig 6&7)
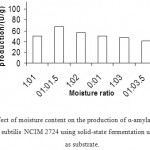 |
Figure 6: Effect of moisture content on the production of α-amylase(U/g) by Bacillus subtilis NCIM 2724 using solid-state fermentation using rice bran as substrate.
|
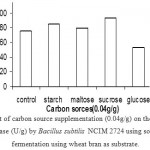 |
Figure 7: Effect of carbon source supplementation (0.04g/g) on the production of α – amylase (U/g) by Bacillus subtilis NCIM 2724 using solid state fermentation using wheat bran as substrate.
|
Effect Of Carbon Source Supplementation On Enzyme Production
Supplementation of carbon sources in the form of mono-saccharides, disaccharides and polysaccharides resulted in marginal increase in a-amylase production by B. subtilis during solid-state fermentation using wheat bran and rice bran. Highest production was observed with glucose and sucrose when supplemented to rice bran and wheat bran respectively. Bacillus thermooleovorans is reported to prefer starch, glucose, lactose, maltose and maltodextrins as carbon sources for a-amylase secretion. In contrast, carbon sources such as glucose, maltose and starch did not enhance α-amylase production by thermophilic B. coagulans in solid-state fermentation using wheat bran. (Fig 8 & 9)
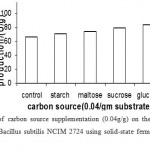 |
Figure 8: Effect of carbon source supplementation (0.04g/g) on the production of α-amylase (U/g) by Bacillus subtilis NCIM 2724 using solid-state fermentation using rice bran as substrate.
|
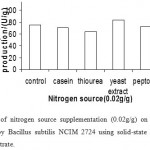 |
Figure 9: Effect of nitrogen source supplementation (0.02g/g) on the production of α-amylase (U/g) by Bacillus subtilis NCIM 2724 using solid-state fermentation using wheat bran as substrate. |
Effect Of Nitrogen Source Supplementation On Enzyme Production
Addition of organic nitrogen sources such as casein, peptone and thiourea to the medium resulted in considerable decrease in α -amylase production by B. subtilis. Supplementation of additional nitrogen sources in general has been reported to be inhibitory for α -amylase production by microorganisms. But in our study addition of yeast extract to rice bran and wheat bran enhanced the enzyme production. Presence of organic nitrogen sources, urea and peptone, has been reported to enhance a-amylase enzyme production by Aspergillus niger in wheat bran containing solid substrate medium, but inorganic nitrogen source, ammonium chloride, repressed enzyme production. Decrease in a-amylase enzyme production during solid-state fermentation when using organic nitrogen sources like casein, gelatin and soy meal as medium supplements has been reported. (Fig 10 & 11)
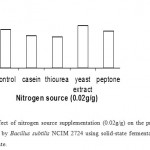 |
Figure 10: Effect of nitrogen source supplementation (0.02g/g) on the production of α-amylase (U/g) by Bacillus subtilis NCIM 2724 using solid-state fermentation using rice bran as substrate.
|
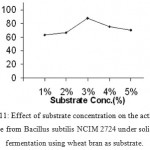 |
Figure 11: Effect of substrate concentration on the activity of amylase from Bacillus subtilis NCIM 2724 under solid-state fermentation using wheat bran as substrate.
|
Effect Of Substrate Concentration On Enzyme Production
Enzyme activity was observed at different substrate concentrations. There was a drastic increase in the enzyme activity with increase in starch concentration from 1.0 to 3.0%. Further increase in substrate concentration has shown decrease in enzyme activity.
(Fig 12 & 13)
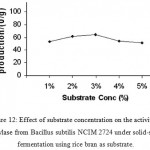 |
Figure 12: Effect of substrate concentration on the activity of amylase from Bacillus subtilis NCIM 2724 under solid-state fermentation using rice bran as substrate.
|
Effect Of Ph On Enzyme Production
Maximum enzyme activity was observed at pH 8.0. Further increase in pH has shown decrease in amylase activity. α-amylase of Bacillus sp. AS-1 is reported to have pH optimum at pH=6.5 and 98 % peak activity at pH=6 and 7. (Fig 14 & 15)
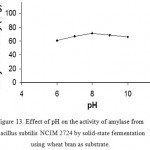 |
Figure 13: Effect of pH on the activity of amylase from Bacillus subtilis NCIM 2724 by solid-state fermentation using wheat bran as substrate.
|
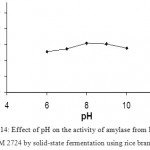 |
Figure 14: Effect of pH on the activity of amylase from Bacillus subtilis NCIM 2724 by solid-state fermentation using rice bran as substrate.
|
Effect Of Temperature On Enzyme Production
As starch liquefaction is generally carried out at higher temperatures of 70–90 °C,
the thermostable α-amylases are of great significance (19). In this study α -amylase produced by B.subtilis showed considerable enzyme activity in the ranges from lower to higher temperature. At 60°C high enzyme activity was observed.
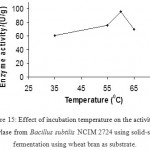 |
Figure 15: Effect of incubation temperature on the activity of amylase from Bacillus subtilis NCIM 2724 using solid-state fermentation using wheat bran as substrate
|
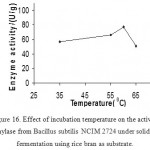 |
Figure 16: Effect of incubation temperature on the activity of amylase from Bacillus subtilis NCIM 2724 under solid-state fermentation using rice bran as substrate.
|
Effect Of Time Interval On Enzyme Production
Enzyme activity was observed at different time periods. The time required for the enzyme to degrade starch was studied. Enzyme has shown highest activity when the incubation time is 15 minutes. Increase in incubation period has shown no increase in the activity of enzyme. Enzyme activity is constant after 15 min time of incubation. (Fig 18 &19)
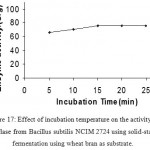 |
Figure 17: Effect of incubation temperature on the activity of amylase from Bacillus subtilis NCIM 2724 using solid-state fermentation using wheat bran as substrate.
|
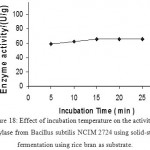 |
Figure 18: Effect of incubation temperature on the activity of amylase from Bacillus subtilis NCIM 2724 using solid-state fermentation using rice bran as substrate.
|
Effect Of Mutants On Enzyme Production
The mutated bacillus species were tested for enzyme production in rice bran and wheat bran. The mutated bacillus species resulting from mutation by acrylamide (5mg/ml for 10% inoculum) has shown increased amylase production than the original strain.
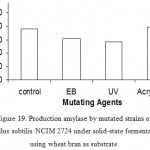 |
Figure 19: Production amylase by mutated strains of Bacillus subtilis NCIM 2724 under solid-state fermentation using wheat bran as substrate.
|
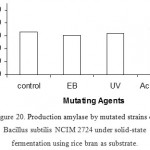 |
Figure 20: Production amylase by mutated strains of Bacillus subtilis NCIM 2724 under solid-state fermentation using rice bran as substrate.
|
Bibliography
- Aguilar,G., Morlon-Guyot, J.,Trejo-Aguilar, B. and Guyot, J.P. Purification and characterization of an extracellular a-amylase produced by Lactobacillus manihotivorans LMG 18010T, an amylolytic lactic acid bacterium, Enzyme Microb. Technol. 27 (2000) 406–413.
- Aiyer, P.V.D., Effect of C:N ratio on alpha amylase production by Bacillus licheniformis SPT 278, Afr. J. Biotechnol. 3 (2004) 519–522.
- ANTO, H., et al., α-Amylase Production by cereus, Food Technol. Biotechnol. 44 (2) 241–245 (2006)
- Baysal, Z., Uyar, F.and Aytekin, C, Solid-state fermentation for production of a-amylase by a thermotolerant Bacillus subtilis from hot-spring water, Process Biochem. 38 (2003) 1665–1668.
- Ben, AM., Mezghani, M. and Bejar, S. (1999). A thermostable α-amylase producing maltohexaose from a new isolated Bacillus US100: study of activity and molecular cloning of the corresponding agent. Enzyme. Microbe. Techno l. 24:548-9.
- Bunni, L., Mc Hale, L. and Mc Hale, A.P. Production, isolation and partial characterization of an amylase system produced by Talaromyces emersonii CBS 814.70, Enzyme Microb. Technol. 11 (1989) 370–375.
- Chandra, A.K., Medda, S. and Bhadra, A.K. Production of extracellular thermostable a-amylase by Bacillus licheniformis, J. Ferment. Technol. 58 (1980) 1–10.
- Coronado, M., Vargas, C., Hofemeister, J., Ventosa, A. and Nieto, JJ. (2000). Production and biochemical characterization of an α-amylase from the moderate halophile Halomonas meridiana. FEMS Microbiol. Lett.183:67-71.
- Dettori, B.G., Priest, F.G. and Stark, J.R. Hydrolysis of starch granules by the amylase from Bacillus stearothermophilus NCA 26, Process Biochem. 27 (1992) 17–21.
- Francis, F., Sabu, A., Nampoothiri, K.M., Ramachandran, S., Ghosh, S., zakacs,G. and Pandey, A. Use of response surface methodology for optimizing process parameters for the production of a-amylase by Aspergillus oryzae, Biochem. Eng. J. 15 (2003) 107–115.
- Goto,C.E., Barbosa, E.P., Kistner, L.C.L., Moreira, F.G. Lenartovicz, V. and Peralta, R.M. Production of amylase by Aspergillus fumigatus utilizing a-methyl-D-glucoside, a synthetic analogue of maltose, as substrate, FEMS Microbiol. Lett. 167 (1998) 139–143.
- Goyal, N., Gupta, J.K. and Soni, S.K. A novel raw starch digesting thermostable a-amylase from Bacillus I-3 and its use in the direct hydrolysis of raw potato starch, Enzyme Microb. Technol. 37 (2005) 723–734.
- Hamilton, L.M., Kelly, C.T. and Fogarty, W.M. (1999). Purification and properties of the raw starch degrading α-amylase of Bacillus IMD434. Biotechol. Lett. 21:111-5.
- Hamilton, L.M., Fogarty, W.M. and Kelly, C.T. Purification and properties of the raw starch degrading a-amylase of Bacillus IMD 434, Biotechnol. Lett. 21 (1999) 111–115.
- Hesseltine, C.W. Biotechnology report. Solid state fermentations. Biotechnol Bioeng. 1972 Jul;14(4):517–532.
- Iefuji, H., Chino, M., Kato, M.and Iimura, Y. Raw starch-digesting and thermostable a-amylase from the yeast Cryptococcus S-2: Purification, characterization, cloning and sequencing, Biochem. J. 318 (1996) 989–996.
- Jensen, B. and Olsen, J. Physicochemical properties of a purified alpha amylase from the thermophilic fungus Thermomyces lanuginosus, Enzyme Microb. Technol. 14 (1992) 112–116.
- Khoo, S.L., Amirul, A.A., Kamaruzaman, M., Nazalan, N. and Azizan, M.N. (1994). Purification and characterization of α-amylase from Aspergillus flavus. Folia Microbiol. 39:392- 398.
- Kunamneni, A., Singh, S., Response surface optimization of enzymatic hydrolysis of maize starch for higher glucose production, Biochem. Eng. J. 27 (2005) 179–190.
- Mendu, D.R., Ratnam, B.V.V., Purnima, A. and Ayyanna, C. Affinity chromatography of a-amylase from Bacillus licheniformis, Enzyme Microb. Technol. 37 (2005) 712–717.
- Mielenz, J.R. Bacillus stearothermophilus contains a plasmid- -borne gene for a-amylase, Proc. Natl. Acad. Sci. 80 (1983) 5975–5979.
- Mishra, S., Noronha, S.B. and Suraishkumar, G.K. Increase in enzyme productivity by induced oxidative stress in Bacillus subtilis cultures and analysis of its mechanism using microarray data, Process Biochem. 40 (2005) 1863–1870.
- Moller, K., Sharif, M.Z. and Olsson, L. Production of fungal a-amylase by Saccharomyces kluyveri in glucose-limited cultivations, J. Biotechnol. 111 (2004) 311–318.
- Morgan, F.J. and Priest, F.G. (1981). Characterization of a thermostable α- amylase from Bacillus licheniformis J. Appl. Bacteriol. 50:107-114
- Mukhopadhyay, R.P. and Chandra, A.L. in Solid State Fermentation (ed. Pandey, A.), Wiley Eastern Publishers, New Delhi, 1994, pp. 142–144
- Najafi, M.F. and Kembhavi, A. One-step purification and characterization of an extracellular a-amylase from marine Vibrio , Enzyme Microb. Technol. 36 (2005) 535–539.
- Ramachandran, S., Patel, A.K., Nampoothiri, K.M., Francis, F., Nagy, V., zakacs, G. and Pandey, A. Coconut oil cake – A potential raw material for the production of a-amylase, Bioresour. Technol. 93 (2004) 169–174.
- Ramesh, M.V. and Lonsane, B.K. Critical importance of moisture content of the medium in alpha amylase production by Bacillus licheniformis M27 in a solid-state fermentation medium, Appl. Microbiol. Biotechnol. 33 (1990) 501–505.
- Robyt, J. and Ackerman, R.J. (1971). Isolation, purification and characterization of a maltotetraose producing amylase from Pseudomonas stutzeri. Biochem. Biophys. 145:105-14.
- Saito, N. (1973). Thermophilic extracellular α-amylase from Bacillus licheniformis. Arch Biochem. Biophys. 155:290- 298.
- Shigechi, H., Fujita, Y., Koh, J., Ueda, M., Fukuda, H. and Kondo, A. Energy saving direct ethanol production from low-temperature cooked corn starch using a cell-surface engineered yeast strain co-displaying glucoamylase and a-amylase, Biochem. Eng. J. 18 (2004) 149–153.
- Sivaramakrishnan, S., t al., α-Amylases from Microbial Sources, Food Technol. Biotechnol. 44 (2) 173–184 (2006)
- Sodhi, H.K., Sharma, K., Gupta, J.K. and Soni, S.K. Production of a thermostable α-amylase from Bacillus PS-7 by solid- state fermentation and its synergistic use in the hydrolysis of malt starch for alcohol production, Process Biochem. 40 (2005) 525–534.
- Srivastava, R.A.K. and Baruah, J.N. Culture conditions for production of thermostable amylase by Bacillus stearothermophilus, Appl. Environ. Microbiol. 52 (1986) 179–184.
- Syu, M.J. and Chen, Y.H. A study on the α-amylase fermentation performed by Bacillus amyloliquefaciens, Chem. Eng. J. 65 (1997) 237–247.
- Tanyildizi, M.S., Ozer, D. and Elibol, M. Optimization of α-amylase production by Bacillus using response surface methodology, Process Biochem. 40 (2005) 2291–2296.

This work is licensed under a Creative Commons Attribution 4.0 International License.





