How to Cite | Publication History | PlumX Article Matrix
Prevalence Study of Hepatitis A Virus (HAV) on Jeddah Population
Maher H. S. Babaeer and M. S. H. AL Awfi
Department of Biology, Faculty of Science, King Abdulaziz University, P.O. Box 13312, Jeddah - 21493 Saudi Arabia.
ABSTRACT: Hepatitis A virus (HAV) infection is often a self limiting disease and infects millions of people worldwide every year. HAV infection is one of the most important public health problems all over the world, especially in areas with high prevalence rate of HAV. Seroprevalence of HAV infection shows great differences not only between different countries but also between different regions in the same country. It is inversely related to the level of socioeconomic status, sanitation and personal hygiene. The objective of this study was prevalence of Hepatitis A virus (HAV) on Jeddah population, Saudi Arabia. Also find out the relationship of our result with age, gender and ethnicity. Serum samples were collected from studied population between 2 and 35 or more years of age and stratified into the following age groups: 2 to 5, 6 to 9, 10 to14, 15 to19, 20 to 24, 25 to 29, 30 to 34 and more than 35 year, Serum samples were not available for less than 2 year of age. Serum samples also were stratified according to nationality into Saudi and non Saudi. And according to sex serum samples were stratified into male and female. Prevalence was calculated separately for each age group, for male and female separately, and for studied population as a whole. Sample sizes were calculated to achieve a 95% confidence interval (CI) of approximately ±5% for each age group. The determination of anti – HAV antibodies was carried out by ELISA-test.For all age groups, there was no significant difference between genders regarding anti-HAV seropositivity. The prevalence of IgG antibodies to HAV was 33.1% overall, 33.6% among males and 32.5% among females with no statistically significant difference (P>0.05). However, it increased significantly with the age. The IgG antibodies to HAV were found in 20.3 % of Saudi population, and 49% of non Saudi population (p<0.05). There was significant difference between nationality regarding anti-HAV seropositivity. The prevalence of HAV among adult population (39.3%) was higher than among children population (22.4%). The chi-square test showed significant differences in the prevalence of HAV between adult and children population (p<0.05).
KEYWORDS: Hepatitis A Virus; Public health problems; Jeddah
Download this article as:| Copy the following to cite this article: Babaeer M. H. S, Awfi M. S. H. A. Prevalence Study of Hepatitis A Virus (HAV) on Jeddah Population. Biosci Biotech Res Asia 2011;8(2) |
| Copy the following to cite this URL: Babaeer M. H. S, Awfi M. S. H. A. Prevalence Study of Hepatitis A Virus (HAV) on Jeddah Population. Biosci Biotech Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9439/ |
The hepatitis A virus (HAV) , a member of the family Picornaviridae, is a nonenveloped virus with a linear, single-stranded, positive-sense RNA genome of approximately 7.5 kb in length (Hu &Arsov, 2009) .There is only one HAV serotype, which is organized into six genotypes (Hollinger and Emerson, 2001). The virus is hardy, surviving on human hands and fingertips and requiring temperatures higher than 185°F (85°C) for inactivation.Hepatitis A virus survives for extended periods in seawater, fresh water, wastewater, and soil (Todd et al., 2009).
HAV is transmitted almost exclusively by the fecal-oral route, and transmission is enhanced by poor hygiene, overcrowding, and contaminated food or water. The presence and severity of symptoms with hepatitis A virus infection is related to the patient’s age. Approximately 70 percent of infected adults develop symptoms, including jaundice. In contrast, only 30 percent of children younger than six years of age develop symptoms, which usually are nonspecific and flu-like without jaundice (Atkinson, 2005).
Although hepatitis A virus (HAV) infection is usually self-limited, it may induce fulminant hepatitis (Park et al, 2010). After faecal–oral transmission of the virus, the infection is associated with the shedding of large amounts of virus from the bowel during the incubation period. Virus shedding is accompanied by viremia for several weeks before and after the onset of clinical symptoms (Cristina and Costa-Mattioli, 2007).
The incubation period is 15 to 50 days, with an average of 25 to 30 days. Common symptoms include malaise, loss of appetite, nausea and icterus. Symptoms, which are often mild and even subclinical, usually resolve within two to four weeks of onset (Heymann, 2004). Current or recent infection can be determined by the presence of anti-HAV IgM antibodies in serum, which are detectable soon after infection and can remain detectable for about 6 months (or longer, in some cases). Past infection can be determined by the presence of anti-HAV IgG antibodies in serum, which appear shortly after the onset of symptoms and present throughout the person’s lifetime, conferring immunity (Jung et al., 2009)
Material and Methods
Serum samples collection
A total of 1050 serum samples; 581 Saudi samples and 469 non Saudi samples were collected from some diagnostic laboratories throughout Jeddah. These laboratories supplied remnant sera from samples that had been submitted for serological testing and would otherwise have been discarded. Sera from subjects who were known to be infected with human immunodeficiency virus, hepatitis B virus and hepatitis C virus were excluded. Sera were identified at the referring laboratory by the sex of the subject, age or date of birth, date of collection, and nationality. The samples were coded by date of collection, nationality/territory of origin, sample number and referring laboratory. All serum samples were stored at -20OC until use.
Study population
Serum samples were collected from studied population between 2 and 35 or more years of age and stratified into the following age groups: 2 to 5, 6 to 9, 10 to14, 15 to19, 20 to 24, 25 to 29, 30 to 34 and more than 35 year, Serum samples were not available for less than 2 year of age. Serum samples also were stratified according to nationality into Saudi and non Saudi. And according to sex serum samples were stratified into male and female. Prevalence was calculated separately for each age group, for male and female separately, and for studied population as a whole. Sample sizes were calculated to achieve a 95% confidence interval (CI) of approximately ±5% for each age group.
Serological testing
Serum samples were tested for HAV-specific immunoglobulin G (IgG) using a HAV IgG enzyme-linked immunosorbent assay (ELISA) technique using DiaSorin kit.
Statistical analysis
The percentages of individuals with positive, negative, and equivocal results were determined for each age group and sex. SPSS program version 18 was used for the analysis and comparison of sero-statuses among age groups, male and female and each nationality. Ninety-five-percent confidence intervals were calculated where appropriate, and P values of < 0.05 were considered statistically significant.
Results
A total of 1050 cases (559 male and 491 female) between ages of 2 years and over 35 years were enrolled in the study. The demographic characteristic of population tested was age, sex, race and nationality (Saudi and non Saudi) as shown in Table 1. The prevalence of IgG antibodies to HAV was 33.1% overall, 33.6% among males and 32.5% among females with no statistically significant difference (P>0.05). These were shown at Table 1. and Fig. 1.
Table 1: Seroprevalence of anti-hepatitis A virus (HAV) Ig G antibodies among studied population in relation to demographic variables.
| P Value | Positive | No. | Age group |
| % No. | |||
| Age (years) | |||
| <0.05 | 17 21 | 124 | 2 – 5 |
| 21.1 28 | 128 | 6 – 9 | |
| 28.8 38 | 132 | 10-14 | |
| 27.2 37 | 136 | 15-19 | |
| 34.3 46 | 134 | 20-24 | |
| 38.2 50 | 131 | 25-29 | |
| 47.7 63 | 133 | 30-34 | |
| 49.2 65 | 132 | >35 | |
| Sex | |||
| >0.05 | 33.6 188 | 559 | Males |
| 32.5 160 | 491 | Females | |
| <0.05 | Nationality | ||
| Saudi | |||
| 25.6 76 | 297 | Males | |
| 51.1 43 | 284 | Females | |
| 20.3 118 | 581 | Total | |
| Non Saudi | |||
| 42.7 112 | 262 | Males | |
| 56.5 117 | 207 | Females | |
| 49 230 | 469 | Total |
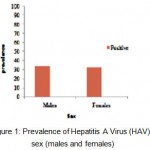 |
Figure 1: Prevalence of Hepatitis A Virus (HAV) by sex (males and females).
|
Table 1. and Fig. 2. show prevalence of Hepatitis A virus by nationality. The IgG antibodies to HAV were found in 20.3 % of Saudi population, and 49% of non Saudi population (P<0.05). There was significant difference between nationalities regarding anti-HAV seropositivity.
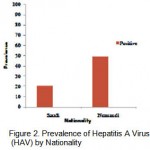 |
Figure 2: Prevalence of Hepatitis A Virus (HAV) by Nationality.
|
The prevalence of HAV among adult population (39.3%) was higher than among children population (22.4%). The chi-square test showed significant differences in the prevalence of HAV between adult and children population (p<0.05). These were shown at Table 1. and Figure 3.
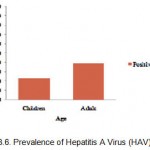 |
Figure 3: Prevalence of Hepatitis A Virus (HAV) by age.
|
Total anti-HAV seroprevalence was significantly different between age groups (P<0.05). HAV prevalence rates according to age groups were as follows: Group 1; 17% (95% CI: 15.8% – 21.4%), Group 2; 21.9%(95% CI: 16.5% – 26.1%), Group 3; 28.8% (95% CI: 25.1% – 32.3%), Group 4; 27.2% (95% CI: 25.5% – 28.9%), Group 5; 34.3% (95% CI: 25.2% – 44.6%), Group 6; 38.2%(95% CI: 33.1% – 43.5 %), Group 7; 47.7 %(95% CI: 42.7% – 52.5%), Group 8; 49.2%(95% CI: 46.1% – 52.5%). The prevalence of HAV by age groups were shown at Table 1.. and figure 4.
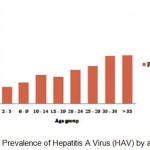 |
Figure 4: Prevalence of Hepatitis A Virus (HAV) by age group.
|
Discussion
Hepatitis A virus (HAV) is a major global public health problem especially in developing countries(Hollinger& Ticehurst; 1996). In these countries, most infections occur under the age of 10 and majority of these infections are asymptomatic (Sherlock & Dooley, 2002). In contrast, the seroprevalence in several industrialized countries in pediatric ages are low and infection is usually acquired during late adolescence and early adulthood and accompanies with significant morbidity (CDC, 2005).
In our study, the enzyme linked immunosorbent assay was used for the detection of HAV IgG in studied population from different nationalities (Saudi and non Saudi) in Jeddah city. The seroprevalence of HAV IgG was 33.1% in studied population. The prevalence rate suggestive of ubiquitous past exposure to infection. This perhaps could be related to socio-economic, environmental, and climatic factors.The results of our study are generally consistent with those of other reports from other countries where HAV infection occurs early in life. Ataei et al found that the seroprevalence for anti-HAV IgG in children over 6 years from urban and rural areas of the Isfahan (Borkhar and Meimeh) was 28.6% (Ataei et al., 2008), Alkhalidi et al reported that the seroprevalence of HAV among healthy outpatients applying for civilian or military jobs in Kuwait was 28.8% (Alkhalidi et al., 2009). The results of our study are different with those of other reports from other countries and this perhaps due to the influence of many factors, such as hygienic circumstances, ethnic and socioeconomic factors, and sexual contacts. Ahmed et al found that the prevalence of HAV in Bangladesh was 74.8% (Ahmed et al., 2009), Gadgil reported in the Indian population indicating a rise in the rate of hepatitis A infection among adults. Positive samples were tested for HAV-RNA. Age wise anti- HAV positivity was significantly low in adults aged 18-25 years (90.4%) compared to those aged >25 years (97.4%) (Gadgil et al., 2008 ).
In most developed countries, the age-specific prevalence curve of anti-HAV has a sigmoid shape, with a low prevalence among children and adolescents and a high prevalence among elderly due to improvements in standard of living and sanitation (Gust, 1992).
Similar previous studies were carried out in different regions of KSA. These studies showed a significant decrease in seroprevalence of anti-HAV across all age groups In majority endemicity for HAV shifted from high or intermediate to intermediate or low over the time period (Tufenkeji, 2000). Study in Riyadh involved children aged up to 15 years reported that by 11 years of age, only 45% of them were anti-HAV-positive, indicating intermediate endemicity in this city (Al-Mazrouet al, 1997). Another study also in Riyadh (urban area) revealed 24.7% of children were seropositive to HAV by age of 12 years(Arif, 1996). Another study in Western of Saudi Arabia that carried out on random samples of schools located at different regions in Jeddah reported that the overall seropositivity was 28.7% (Jaber, 2006). The above studies and the present one indicate that pattern of endemicity of HAV infection is shifting in KSA (Tufenkeji, 2000). On contrary, seroprevalence study in Eastern region of KSA (Dammam) showed that hepatitis A virus infection starts dramatically high in school-age children, and then rise gradually with an increase in age reflecting high endemic of HAV in this region (Fathallaet al, 2000).
Hepatitis A vaccines provide highly immunogenic, safe, and long-lasting protection (Andre et al, 2002). Annual incidence of hepatitis A has dropped markedly after introducing inactivated hepatitis A vaccine into the national childhood vaccination programme in endemic areas (Chodicket al, 2008). The availability of vaccines and their widespread use will provide an opportunity to lower the overall incidence of hepatitis A disease. According to our study, the use of the vaccine should be encouraged in Saudi children. Further studies can be carried out to make solid recommendation of the vaccine in other groups at risk.
References
- Ahmed, M., Munshi, S.U., Nessa, A., Ullah, M.S., Tabassum, S. and Islam M.N., High prevalence of hepatitis A virus antibody among Bangladeshi children and young adults warrants pre-immunization screening of antibody in HAV vaccination strategy, Indian Journal of medical microbiology, 27: 48-50 (2009).
- Alkhalidi, J., Alenezi, B. and Al-Mufti S., Seroepidemiology of hepatitis A virus in Kuwait, World Journal Gastroenterology, 15: 102-105 (2009).
- Al-Mazrou Y, Khalil M, Al-Jeffri M, Al-Howasi M. Serosurvey of hepatitis A (HAV IgG positive) among children in Riyadh, Saudi Arabia. Kingdom of Saudi Arabia: Ministry of Health (1997).
- Andre, F., Van Damme, P., Safary, A., and Banatvala, J., Inactivated hepatitis A vaccine: immunogenicity, efficacy, safety and review of official recommendations for use, Expert Review of Vaccines,1: 9-23 (2002).
- Arif, M., Enterically transmitted hepatitis in Saudi Arabia: an epidemiological study. Annals of Tropical Medicine and Parasitology, 90:
197-201 (1996). - Ataei, B., Javadi, A.A. and Nokhodian Z., HAV in Isfahan province: a population-based study, Tropical Gastroenterology, 29: 160-162 (2008).
- Atkinson W. Hepatitis A. Epidemiology and Prevention of Vaccine-Preventable Diseases. 8th ed. Atlanta, Ga.: Centers for Disease Control and Prevention, 177-89, A11, A33-4 (2005).
- CDC., Positive test results for acute hepatitis A virus infection among persons with no recent history of acute hepatitis. MMWR. 54: 453-6 (2005).
- Chodick, G., Heymann, A.D., Ashkenazi, S., Kokia, E. and Shalev, V., Long-term trends in hepatitis A incidence following the inclusion of hepatitis A vaccine in the routine nationwide immunization program, Journal of Viral Hepatitis, 15: 62-65 (2008).
- Cristina, J. and Costa-Mattioli, M., Genetic variability and molecular evolution of hepatitis A virus. Virus Research, 127: 151-157 (2007).
- Fathalla, S.E., Al-Jama, A.A., Al-Sheikh, I.H. and Islam, S.I., Seroprevalence of hepatitis A virus markers in Eastern Saudi Arabia, Saudi Medical Journal, 21: 945-949 (2000).
- Gadgil, P.S., Fadnis, R.S., Joshi, M.S., Rao, P.S and Chitambar S.D., Seroepidemiology of hepatitis A in voluntary blood donors from Pune, western India (2002 and 2004-2005), Epidemiology and Infection, 136: 406-409 (2008).
- Gust, I.D., Epidemiology patterns of hepatitis A in different parts of the world, Vaccine, 10: 56-58 (1992).
- Heymann, D.L. Viral hepatitis A. In: Heymann DL, ed. Control of Communicable Diseases Manual. 18th ed. Washington, D.C.: American Public Health Association, 247-53 (2004).
- Hollinger, F.B. & Emerson, S.U., Hepatitis A virus. In Fields Virology, 4th edn. (ed. D. M. Knipe, P. M. Howley, D. E.Griffin, R. A. Lamb, M. A. Martin, B. Roizman& S. E. Straus), Lippincott-Raven Publishers, Philadelphia, pp. 799–840 (2001).
- Hollinger, F.B., Ticehurst, J.R. Hepatitis A virus. In: Fields BN, Knipe DM. Howley PM, et a!. editors. Fields Virology. 3rd ed. Philadelphia: Lippincott Raven Publishers: p. 735-82 (1996).
- Hu, Y. and Arsov, I., Nested real-time PCR for hepatitis A detection. Letters in Applied Microbiology, 49: 615e-619 (2009).
- Jaber, S.M., Prevalence of anti-hepatitis B and antihepatitis A antibodies among school aged children in Western, Saudi Arabia, Saudi Medical Journal, 27: 447-456 (2006).
- Jung, S.I. C. S. Lee, K. H., Park, E.S., Kim, Y.J., Kim, G.S., Kim, D.S., Lim, J.E., Moon, J.J., Min, H.S., Bom, M. H., Jung, Y.J., Chang, S.L., Chae and J.H. Lee ., Sero-epidemiology of hepatitis A virus infection among healthcare workers in Korean hospitals, Journal of Hospital Infection, 72: 251-257 (2009).
- Sherlock, S. & Dooley, J. Diseases of the Liver and Biliary System, 11th edition, Blackwell Publishing. 273-6 (2002).
- Todd, E.C., Greig, J.D., Bartleson, C.A. and Michaels B.S., Outbreaks where food workers have been implicated in the spread of foodborne disease. Part 6. Transmission and survival of pathogens in the food processing and preparation environment, Journal of food protection, 72: 202-219 (2009).
- Tufenkeji, H., Hepatitis A shifting epidemiology in the Middle East and Africa. Vaccine, 18:
S65-67 (2000).

This work is licensed under a Creative Commons Attribution 4.0 International License.





