Manuscript accepted on : 31 December 2011
Published online on: --
Yahya A. M. Floos1, Abdulmohsin A. Al-Sofyani2 and Talal A. Zari1
1Department of Biology, King Abdul-Aziz University, Jeddah, Kingdom of Saudi Arabia.
2Department of Marine Biology, King Abdul-Aziz University, Jeddah, Kingdom of Saudi Arab
Corresponding Author E-mail: Yahya_floos2008@yahoo.com
ABSTRACT: Sexual reproduction of the scleractinian corals Seriatopora hystrix and Lobophyllia corymbosa was studied for a period of one year in Sharm Ubhur. They indicated that, S. hystrix is hermaphrodite brooders with internal fertilization and developing the larvae within their polyps. Whereas, the L. corymbosa is hermaphrodite broadcasters with external larval developing, hence embryos of the species are not observed in the histological examinations. The onset of reproductive period of S. hystrix was found to be limited (January to April). The number of eggs and testes observed in this period were also limited and the first planula larvae were observed in the March at temperature 27.42oC. In L. corymbosa, the gonads were found in the polyps throughout the year with two cycles of oogenesis and 1 cycle of spermatogenesis. The S. hystrix egg size was ranged from 158 µm (in January) to 241 µm (in April). The first visible size of eggs of L. corymbosa were observed in August (187.2 µm) and in April (689.96 µm). Zooxanthellae were presented in the mature oocytes in S. hystrix. While in L. corymbosa they were not found.
KEYWORDS:
Sexual reproduction; Reef Corals; Red Sea; Jeddah
Download this article as:| Copy the following to cite this article: Floos Y. A. M, Al-Sofyani A. A, Zari T. A. Sexual Reproduction of Two Reef Building Corals Seriatopora hystrix and Lobophyllia corymbosa in the Jeddah Coast of Red Sea. Biosci Biotech Res Asia 2012;9(1) |
| Copy the following to cite this URL: Floos Y. A. M, Al-Sofyani A. A, Zari T. A. Sexual Reproduction of Two Reef Building Corals Seriatopora hystrix and Lobophyllia corymbosa in the Jeddah Coast of Red Sea. Biosci Biotech Res Asia 2012;9(1). Available from: https://www.biotech-asia.org/?p=9463 |
Introduction
Studies on sexual reproduction of hermatypic corals have increased during the last two decades, with information of about 30% of the known species are only available (Kojis and Quinn 1982; Harriott, 1985, and Harrison and Wallace, 1990).
Corals are commonly hermaphroditic and exhibit two primary types of reproductive modes known as broadcast spawning and brooding (Knowlton, 2001 and Harrison and Wallace, 1990). In broadcast spawning, fertilization and development of larvae is occurred outside of the maternal colony in the water column. This can then result in the widespread dispersal of larvae, since larvae travel with water currents during development.
Brooding corals exhibit a very different reproductive strategy. Only sperms are released into the water column and reach the eggs in the maternal colony. The fertilization is taken place in the maternal colony itself and then developed larvae are released into the water as swimming larvae (Knowlton, 2001). In this mode, the larvae are capable of settling on nearby substrate shortly after released. The number of larvae is limited due to the probability of sperms from one colony to reach the eggs of another colony for the successful reproduction. Therefore, reef degradation may be occurred not only because of the lowered gamete production, but also due to the reduced fertilization rate of those gametes released.
Seriatopora hystrix is a hermaphroditic brooding coral widely distributed from the Red Sea to the Western Pacific Ocean (Veron, 2000). It has sexually produced larvae (Ayre and Resing 1986; Sherman, 2008) that are matured within the polyps and periodically released into the water column (Harrison and Wallace, 1990). Most brooding corals, including S. hystrix have larvae competent to settle within a few hours after release (Harii et al, 2002). Recent work on S. hystrix population using highly variable microsatellite markers showed that the dispersal mostly occurred over small spatial scales (<100 meters), supplemented by less frequent longer distance dispersal (Underwood et al, 2007). Despite the usual rapid settlement of larvae, some brooding corals produce a small proportion of larvae that have extended competency periods of over 100 days in the water column (Harii et al, 2002), which can translate to a dispersal potential of hundreds of kilometers (Harrison and Wallace 1990, Harrison and Booth 2007). Larval competency period is one of the most significant factors affecting dispersal distance (Bohonak 1999, Shanks, 2009), in addition to other biotic and abiotic factors (Harrison and Wallace 1990, Harrison and Booth, 2007).
The coral larvae travel distance can be affected by the reproductive mode of their parental colonies. This is because of different mean larval competency periods resulting from brooding versus broadcast spawning coral species (Harrison and Wallace 1990, Harrison and Booth, 2007). Brooding coral species can develop larvae internally by either sexually or asexually and these larvae can often settle immediately or shortly after release from the parental colony (Atoda 1947, Richmond 1987, Harii et al, 2002). Settlement and genetic data support routine dispersal distances of brooding coral larvae in the range of 10-100s of meters (Tioho et al, 2001, Underwood et al, 2007). Conversely, larvae formed by broadcast spawning and external fertilization have a minimum competency period of > 24 hours (Miller and Mundy 2003, Nozawa and Harrison 2005, Harrison 2006), which aids in dispersing propagates away from parental colonies. The aim of this study is to examine the reproduction mode and timing of S. hystrix and L. corymbosa.
Material and Methods
The study was carried out in the onshore laboratory of the Faculty of Marine Science, King Abdulaziz University, Jeddah, which is 35 km away from the north of the city and located adjacent to the fringing reefs of the Ubhur Creek (Fig. 1). The study site was located in the northern side mouth of the Creek. The optimum water depth of the study site was 5m. Water temperature was measured in situ with two minimum and maximum mercury thermometers attached to the reef in a shaded location.
For reproductive study, samples of S. hystrix and L. corymbosa were taken at every fortnight for the period of one year from January 2009 to December 2009 at 5m depth. Coral specimens were cut off from the coral colony using Chisel and Hammer then fixed in a container with 7% seawater formalin for three days. Thereafter, they were transferred to 10% solution of nitric acid to decalcify the skeleton. The decalcified polyps were washed then with distilled water and preserved in the solution of 70 % ethanol for further histological study. The preserved polyps were passed through a series of ethanol concentration from 70% ethanol to absolute alcohol and then cleared with xylene (Humason , 1979) .The cleared specimens were infiltrated and imbedded in pure paraffin (melting point 58-60°C). Serial sections with a thickness of 7 μm were cut through the polyps using a microtome, and then stained with haematoxylin and eosin. Thereafter, the stained sections were cleared with xylene and mounted in Canada balsam. The gonad development stages (eg. testis and eggs) were measured by a calibrated eyepiece micrometer of compound light microscope.
 |
Figure 1: Red Sea map and location of Ubhur Creek, which lies in north of Jeddah city. The study site is marked as (•) at the entrance of Ubhur Creek.
|
Results
Temperature
The monthly mean temperature together with the maximum and minimum temperatures of each month is shown (Fig.2). The lowest temperature was (27.42±0.80°C) observed in March 2009 While, the highest was (32.67±0.29°C) observed in August 2009. The mean difference between the maximum and minimum temperature was 2.5°C. The annual range of monthly mean temperature was between 27.42±0.80 and 32.67±0.29 with a difference of 5.25°C.
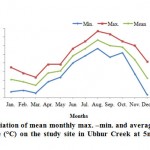 |
Figure 2: Variation of mean monthly max. –min. and average sea water temperature (°C) on the study site in Ubhur Creek at 5m depths, for year 2009.
|
Development of gonads and reproduction period
The histological studies revealed that the both species of S. hystrix and L. corymbosa are hermaphrodite. L. corymbosa is a protogynous hermaphrodite in which female gonads develop earlier than male gonads (Table 1 and 2) and (Figs.3 and 4), whilst S. hystrix is simultaneous hermaphrodite where female and male gonads develop at the same period (Table 1) and. (Figs. 3).
Studies on the S. hystrix showed that it has a short span of reproductive season. This was evidenced by the first appearance of small eggs (158.00 ± 50.59 (18) µm) in the January which was gradually increased to the maximum of 241.8 ± 7.30 (16) µm in the April (Figs.6A), (Table 1), whereas in the case of L. corymbosa, the reproductive season span was longer. The first appearance of small eggs (187.20± 45.33 (3) µm in diameter) were noticed in the August (at temperature 32.67oC) (Fig2 an Fig. 8A) and the highest size of eggs (689.96 ± 127.07 (11) µm) were observed in the April (at temperature 28.83oC). Similarly, the development of testes of S. hystrix had noticed in the January and the size was increased progressively until April (Table 1).
In S. hystrix, the ovaries and testes were arranged on different pairs of mesenteries. These mesenteries were further arranged in a stalk. The taller and thinner stalks were observed as males whereas the shorter and thicker were the females. But in the case of L. corymbosa, the male and female gonads were arranged separately within the same mesentery (Fig. 7A and 8A). The sperms of L. corymbosa were spherical in shape in matured testes (Figs. 8B). In the early stage, the nucleus of the eggs was observed in the center, but the nucleuses were further moved towards the
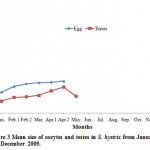 |
Figure 3: Mean size of oocytes and testes in S. hystrix from January 2009 to December 2009.
|
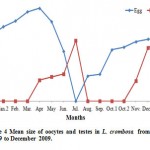 |
Figure 4: Mean size of oocytes and testes in L. crombosa from January 2009 to December 2009.
|
Figure 5 Histological cross-section through polyp of S. hystrix (A), showing zooxanthellae (zx) in the mature oocyte; mesenterial filament (mf) Scale bar = 58.87µm. (B), showing planula with gastrovascular cavity (gvc); measenteries filament (mf) . Scale bar =92.11 µm.
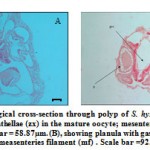 |
Figure 5: Histological cross-section through polyp of S.
|
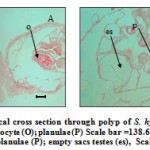 |
Figure 6 Histological cross section through polyp of S. hystrix (A) showing mature oocyte (O); planulae (P) Scale bar =138.67 µm. (B) showing mature planulae (P); empty sacs testes (es), Scale bar = 49.92 µm.
|
Figure 7 Histological section through the polyps of L.crombosa (A) showing female gonads on stalks. St: stalk; O: oocyte. Scale bar =109.9 µm. (B) showing the location of, nu., nucleus; n; nucleolus. Scale bar = 116.38 µm.
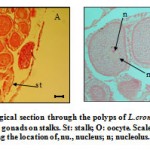 |
Figure 7 Histological section through the polyps of L.crombosa (A) showing female gonads on stalks. St: stalk; O: oocyte.
|
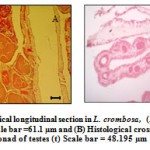 |
Figure 8 Histological longitudinal section in L. crombosa, (A) showing ovaries (O) Scale bar =61.1 µm and (B) Histological cross-section showing a male gonad of testes (t) Scale bar = 48.195 µm.
|
surrounding cell wall while the eggs matured (Fig. 7B). Zooxanthellae were observed in the matured eggs of the S. hystrix (Fig.5A).
In L. corymbosa, since they are hermaphrodite, the planula were not observed in the coelenterons of the polyps. But in the S. hystrix, fertilizations of the eggs were observed within the coelenterons of the polyps. These fertilized eggs may further produced planula through embryogenesis. This was evidenced by the presence of brooding planulae in the coelenterons, beneath the pharynx (Figs. 5B and 6A).
Planulae were observed from March to April reaching a maximum size of 150.8±26.00 (7) µm in April. During this period, differentiation of planula was noticed in the cross section studies of the mesenteries of the developing coelenterons (Figs. 6B)
Discussion
Studies on the reproduction of S. hystrix indicated that the species were hermaphrodite brooders. The fertilization was internal and the larval development was occurred within their polyps. The L. corymbosa were the hermaphrodite broadcaster spawners with external larval development. Hence the embryos of these species were not found during the histological studies (Table 1 and 2) and (Figs. 3 and 4). Throughout the study period, the gonads of L. corymbosa were noticed in the polyps. Two cycles of oogenesis and 1 cycle of spermatogenesis were resulted from this study. Rinkevich and Loya (1979a) suggested that the branching or small-polyped corals are developing gonads in their coelenterons and producing small eggs and brood planulae. While the massive or large-polyped corals are producing many eggs which are spawned for external fertilization. In general, the brooding specie produces few large eggs, in order to provide supplementary nutrition for each planula. Thus, different re-productive modes may have arisen in response to energetic constraints and environmental pressures.
The hermaphrodite brooder reported since in the Red Sea are Seriatopora caliendrum (Fadlallah, 1983 and Shlesinger and Loya, 1985), and Seriatopora hystrix (Maier et al., 2005 and Maier et al., 2009). In worldwide, the Seriatopora hystrix was also reported as brooder in the Great Barrier Reef (Ayre and Dufty 1994; Ayre; Hughes, 2000 and Noreen, 2010) and in the northern part of Okinawa Island, Japan (Nozawa and Loya, 2005). In the present study, S. hystrix showed similar reproductive mode as in Seriatopora caliendrum. The Stylophora pistillata has been also reported as a brooding coral species of the Red Sea (Rinkevi and Loya 1979a, b, Al-Sofyani, 1991).
Similar to S. hystrix, the L. corymbosa of the present study were also reported as hermaphrodite broadcast spawners in the Red Sea (Shlesinger et al, 1998; Robert and Hunter, 1990; Harrison and Wallace, 1990; Hoogenboom, 2008). On the other hand, lot of studies on L. corymbosa from different geographic locations reported that L. corymbosa was a hermaphrodite broadcaster spawner (Riddle, 2008). Differences on the reproductive patterns of S. hystrix and mainly L. corymbosa from different geographic areas were related to differences in many parameters such as colony morphology, polyp size, environmental conditions, habitats, lunar tidal cycle and day-night cycle (Stimson, 1978; Kojis and Quinn, 1981; Rinkevich and Loya, 1979a ; Rinkevich and Loya, 1979b Tomascik and Sander, 1987 and Al-Sofyani, 2008), but none of these studies were given explanation for the root cause of these differences (Fadlallah, 1985).
The onset of reproductive period of S. hystrix was found to be of very short duration i.e. from January to April (Figs. 3), and number of eggs and testes were observed at the end of April (at temperature 28.83oC) were also less (1-2eggs). Planulae of S. hystrix were first observed in the March (at temperature 27.42oC). Harriott (1985) pointed out that planulation in the winter season was common in pocilloporid coral with the correlation of lower light levels of the surface water. The maximum swimming phase of the planulae was lasted three days only, whereas Rinkevich and loya (1979b) found that they would prefer settlement for up to 35 days by enabling the species to be distributed over a long distance (Jokiel 1985; Richmond and Jokiel, 1984).
Meanwhile the oogenesis of L. corymbosa was observed in the August in the study site which was coinciding with seawater temperature (fig. 2). Gametogenesis and spawning may be found different for the same species due to several factors like latitudinal changes (Szmant 1986, Soong 1991, Van Veghel 1994, Acosta and Zea 1997; Knowlton et al, 1997), temperature, day length or both (Babcock, 1995; Fan and Dai, 1995; Fadlallah, 1996; Ben-David-Zaslow et al, 1999), solar energy (Van Veghel 1994), current and wind regimes (Babcock et al, 1986) and rainfall (Acosta and Zea, 1997). The annual reproduction cycle of L. corymbosa was observed as 10 – 11 month period, in which oogenesis was found started in the month of August and was completed in May-June. Moreover, period of the spermatogenesis was lesser with the evidence of the sperm cysts observed in the April month samples only. The gamete releasing was also not observed in the field. The absence of gonads in histological samples in July revealed that the spawning season might be between May 25 (five days after full moon) and June 24.
The egg size of S. hystrix observed in this study was ranged from 158 µm in January to 241 µm in April (Table 1). There are comparative studies on Seriatopora caliendrum and S. hystrix in the Red Sea, the maximum diameter of the eggs was 240 µm (Shlesinger et al., 1998). The first visible egg in L. corymbosa was found during August 187.2 µm to 689.96 µm in April (at temperature 28.83oC). (Table 2) in the highest temperature. Shlesinger et al., (1998) reported similar findings to our studies on Lobophyllia corymbosa, the maximum diameter of the eggs 600 µm and another study stated that the maximum diameter of the eggs was 750 µm (Harrison and Wallace, 1990). Zooxanthellae were presented in the mature oocytes in S. hystrix. While in L. corymbosa they were not found. Similar results were also reported by Sier and Olive (1994) who found zooxanthellae infestation have occurred at a later stage in this case. Harrison and Wallace (1990) reported that, the infestation may occurred weeks before spawning, as found in the genus Porites and Montipora spp or as minimum as 24 hours before spawning in the Montipora digitata.
References
- Acosta, A., Zea, S. Sexual reproduction of the reef coral Montastraea cavernosa (Scleractinia: Faviidae) in the Santa Marta area, Caribbean coast of Colombia. Mar. Biol., 1997; 128: 141-148.
- Al-Sofyani, A. A., Studies on Physiology and ecology of Stylophora pistillata and Echinopora gemmacea from the Red Sea. Ph. D. Thesis. Glasgow. U.K, 1991;pp 167.
- Al-Sofyani, A. A., Reproduction of the red sea corals Pocillopora damicornis and Pocillopora verrucosa in Creek Obhur, Jeddah, Saudi Arabia. Journal of Environmental Sciences. 2008; 36:51-62.
- Atoda, K., The larva and postlarval development of some reef-building corals. I. Pocillopora damicornis cespitosa (Dana). Sci Rep Tohoku Univ Ser., 1947; 4: 24-
- Ayre, D.J., Dufty, S., Evidence for restricted gene flow in the viviparous coral Seriatopora hystrix on Australia’s Great Barrier Reef. Evolution, 1994; 48: 1183-1201.
- Ayre, D.J., Hughes, T.P., Genotypic diversity and gene flow in brooding and spawning corals along the Great Barrier Reef, Australia. Evolution; International Journal of organic Evolution, 2000; 54(5): 1590-1605.
- Ayre, D.J., Resing, J.M., Sexual and asexual reproduction of planulae in reef corals. Marine Biol., 1986; 90:187-190.
- Babcock, R., Synchronous multispecific spawning on coral reefs: potential for hybridization and roles of gamete recognition. Reprod. Fert. Develop., 1995; 7: 943-950.
- Babcock, R.C., Bull, G.D., Harrison, P.L., Heyward, A.J., Oliver, J.k., Wallace, C.C., willis, B.L., Synchronous spawinges of 105 scleractinian coral species on the Great Barrir Reef. Mar.Biol., 1986; 90:379-394.
Y. Ben-David-Zaslow, G. Henning, D.K. Hofmann, Y. Benayahu - Ben-David-Zaslow, Y., Henning, G., Hofmann, D.K., Benayahu, Y., Reproduction in the Red Sea soft coral Heteroxenia fuscescens: seasonality and long-term record (1991 to 1997). Mar. Biol., 1999; 133: 553-559.
- Bohonak, A.J., Dispersal, Gene Flow, and Population Structure. Quaterly Review of Biology, 1999; 74: 21-51.
- Fadlallah, Y.H., Sexual reproduction, development and larval biology in scleractinian corals. A review. Coral Reefs. 1983; 2: 129 – 150.
- Fadlallah, Y.H., Reproduction in the coral Pocillopora verrucosa on the reefs adjacent to the industrial city of Yanbu (Red Sea, Saudi Arabia). In: proc 5th int coral Reef Conger. Antenne Museum- EPHE (Gabrie´ C. ed), Moorea, French Polynesia. 1985; 4:313 – 318.
- Fadlallah, Y.H., Synchronous spawning of Acropora clathrata coral colonies from the western Arabian Gulf (Saudi Arabia). Bull. Mar. Sci., 1996; 59: 209-216.
- Fan, T.Y., Dai. D.F., Reproductive ecology of the scleractinian coral Echinopora lamellosa in the northern and southern Taiwan. Mar. Ecol. Prog. Ser., 1995; 123: 565-572.
- Harii, S., Kayanne, H., Takigawa, H., Hayashibara, T., Yamamoto, M., Larval survivorship, competency periods and settlement of two brooding corals, Heliopora coerulaea and Pocillopora damicornis. Mar Biol., 2002 141, 39-46.
- Harriott, V.J., Recruitment patterns of scleractinian corals at lizard Island, Great Barrier Reef. Proc. 5th Int. coral reef Congress, Tahiti, 1985; 4:367- 372.
- Harrison, P.L., Settlement competency periods and dispersal potential of scleractinian reef coral larvae. Proceedings of the 10th International Coral Reef Symposium, 2006; 78-82.
- Harrison, P.L., Booth, D.J., Coral reefs: naturally dynamic and increasingly disturbed ecosystems. In Marine Ecology (Connell S.D. and Gillanders B.M. ed) Melbourne: Oxford University Press, 2007; pp. 316-377.Harrison, P.L., Wallace, C.C., Reproduction, dispersal, and Hubbard D K (1986) Sedimentation as a control of reef development. Coral Reef, 1990; 5: 117-125.
- Hoogenboom, M. O., Physiological models of performance for scleractiania corals. Ph. D. Thesis. James cook university. Australia, 2008; pp 210.
- Humason, G.L., Animal Tissue Techniques. W.H. Freeman and Company, 4th (ed)., San Francisco, USA, 1979; pp. 23-45.
- Jokiel, P. L., Lunar periodicity of planulae release in the reef coral Pocillopora damicornis in relation to various environmental factors., Great Barrier Reef. Proc. 5th Int. coral reef Congress, Tahiti, 1985 4:307-312.
- Knowlton, N., The future of coral reefs. Proceedings Of The National Academy Of Sciences Of The United States Of America, 2001; 98(10): 5419-5425.
- Knowlton, N., Maté, J.L., Guzmán, H.M.R., Rowan J. Jara., Direct evidence for reproductive isolation among the three species of the Montastraea annularis complex in Central America (Panama and Honduras). Mar. Biol., 1997; 127: 705-711
- Kojis, B. L., Quinn, N. J., Aspects of sexual reproduction and larva development in the shallow water hermatypic coral Goniastrea australensis, Bull. Mar. Sci., 1981; 31: 558 – 574.
- Kojis, B.L., Quinn, N.J., Reproductive ecology of two faviid corals (Coelenterata: Scleractinia). Mar. Ecol. Progr. Ser., 1982; 8: 251-255.
- Maier, E., Tollrian, R., Baruch, R., Nurnberger, B., Isolation by distance in the scleractinian coral Seriatopora hystrix from the Red Sea. Mar. Biol., 2005; 147, 1109-1120.
- Maier, E., Tollrian, R., Nurnberger, B., Fine-scale analysis of genetic structure in the brooding coral Seriatopora hystrix from the Red Sea, Coral Reefs, 2009; 28:751–756.
Miller, K., Mundy, C., Rapid settlement in broadcastspawning corals; implications for larval dispersal. Coral Reefs 22:99–106 - Noreen, A.M.E., Ecological and evolutionaryconnectivity of reef corals in subtropical eastern Australia: implications for the persistence of high-latitude coral populations. Ph.D. Thesis. Southern Cross University, Lismore, NSW. 2010; 145pp.
- Nozawa, Y., Harrison, P. L., Temporal settlement patterns of larvae of the broadcast spawning reef coral Favites chinensis and the broadcast spawning and brooding reef coral Goniastrea aspera from Okinawa, Japan. Coral Reefs, 2005; 24, 272-282.
- Nozawa, Y., Loya, Y., Genetic relationship and maturity state of the allorecognition system affect contact reactions in juvenile Seriatopora corals, Mar. Ecol. Prog. Ser., 2005; 286: 115–123
- Richmond, R.H., Energetic relationships and biogeographical differences among fecundity, growth and reproduction in the reef coral Pocillopora damicornis. Bull Mar Sci., 1987; 41:594-604.
- Richmond, R.H., Jokiel, P. L., Lunar periodicity in larva release in the Reef Coral Pocillopora damicornis at Enewetak and Hawaii. Bull. Mar. Sci. 1984; 34: 280-287.
- Riddle, D., Coral reproduction, parte Three: Stony Coral Sexuality, Reproduction Mode, Puberty Size, Sex Ratios and life Spans 1: Memoriam coral Reproduction, parte3. Micro-Ecosystem Total organic carbon, (Wilkens, P. ed.) part2 Elsevier, A reefs.org publication, 2008VII, Issue IX
- Rinkevich, B., Loya, Y., The reproduction of the Red Sea coral Stylophora pistillata. 1. Gonads and planulae. Mar. Ecol. Prog. Ser., 1979a; 1: 133 -144.
Rinkevich, B., Loya, Y., The reproduction of the Red Sea coral Stylophora pistillata. 11. Synchronization in breeding and seasonality in planulae shedding. Mar. Ecol. Prog. Ser., 1979b; 1: 145 – 152. - Robert, H.R., Hunter, C. L., Reproduction and recruitment of corals comparisons among the Caribbean, the Tropica Pacific, and the Red Sea. Mar. Ecol. Prog. Ser.1990; 60: 185-203.
- Shanks, A.L., Pelagic Larval Duration and Dispersal Distance Revisted. Biol. Bull., 2009; 216: 373-385.
- Sherman, C.D.H., Mating system variation in the hermaphroditic brooding coral, Seriatopora hystrix. Heredity. 2008; 100: 296-303.
- Shlesinger, Y., Goulet T.,L., Loya, Y., Reproductive patterns of scleractinian corals in the northern Red Sea. Mar. Biol., 1998; 132: 691-701
- Shlesinger, Y., Loya, Y., Coral community reproductive patterns: Red Sea versus the Great Barrier Reef. Science, 1985; 228: 1333 – 1335.
- Sier, C.J.S., Olive, P.J.W., Reproduction and reproductive variability in the coral Pocillopora verrucosa from the Republic of Maldives. Mar. Biol., 1994; 118: 713-722.
- Soong, K. (1991). Sexual reproductive patterns of shallow- water reef corals in Panama. Bull. Mar. Sci. 49: 832-846.
- Stimson, J. S. (1978). Mode and timing of reproduction in some common hermatypic corals of Hawaii and Enewetak. Mar. Biol., 48: 173 – 184.
- Szmant, A. M., Reproductive ecology of Caribbean reef corals. Coral. Coral Reef, 1986; 5: 43-54
- Tioho, H., Tokeshi, M., Nojima, S., Experimental analysis of recruitment in a scleractinian coral at high latitude. Mar. Ecol. Prog. Ser. 2001; 213: 79-86.
- Tomascik, T. and Sander, F. (). Effects of eutrophication on reef-building corals I11. Reproduction of the reef building coral Porites porites Mar. Boil. 1987; 94: 77 – 94.
- Underwood, J.N., Smith, L.D., Van Oppen, M.J.H., Gilmour, J.P., Multiple scales of genetic connectivity in a brooding coral on isolated reefs: implications for managing resilience. Ecol Appl 2007; 19: 18-29.
- Van Veghel, M.L.J., Reproductive characteristics of the polymorphic Caribbean reef building coral Montastraea annularis. I. Gametogenesis and spawning behavior. Mar. Ecol. Prog. Ser. 1994; 109: 209-219.
- Veron, J.E.N., Coral of the world. Australian Institute of marine Science and CRR Qld pty ltd Townsvill, (Ed. Stafford-Smith, M.).,2000;vol 2: pp 429.

This work is licensed under a Creative Commons Attribution 4.0 International License.





