How to Cite | Publication History | PlumX Article Matrix
Strain Improvement Studies on T. Thalpophilus strain KS-05
K.Sreenivasa Rao¹*, P. Ellaiah² and Karnakumar¹
¹RRKS,s College of Pharmacy, Bidar, Karnataka India.
²College of Pharmaceutical Sciences, Andhra University, Visakhapatnam, Andhra Pradesh India.
ABSTRACT: The strains of Thermoactinomyces thalpophilus KS-05 was subjected to strain improvement program with a view to obtain increased amylase production. The most effective mutagens, Ultra Violet rays (UV) and Ethyl Methyl Sulphonate (EMS), were chosen for strain improvement. The results indicated that among UV mutants, KSR 14, KSR 17 & KSR 22 showed enzyme yields of 1924, 1986 and 1998 U/ml respectively .All these three mutants were further subjected to EMS treatment and a total of 21 mutants were selected and tested for their amylase producing capacities. The results indicated that the mutant KSV 17 derived from the parent culture KS-05 was the highest amylase yielding strain (2476.6 U/ml). The UV mutation resulted in a mutant KSR 22 with 1998 U/ml productivity which is 13.5% higher production than parent strain. The chemical mutagen (EMS) yielded a better amylase producing mutant KSV 17 with 2476.6 U/ml. Thus the strain improvement programme resulted in a mutant that produced 2.19 times higher titres of amylase over the parent wild strain (1128 U/ml), which is a significant increase of yield.
KEYWORDS: Thermoactinomyces thalpophilus; Ethyl Methyl Sulphonate (EMS); Ultra violet irradiation (UV)
Download this article as:| Copy the following to cite this article: Rao K. S, Ellaiah P, Karnakumar. Strain Improvement Studies on T. Thalpophilus strain KS-05. Biosci Biotech Res Asia 2011;8(2) |
| Copy the following to cite this URL: Rao K. S, Ellaiah P, Karnakumar. Strain Improvement Studies on T. Thalpophilus strain KS-05. Biosci Biotech Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9508/ |
Introduction
Strain improvement is an essential part of process development for fermentation products. Developed strains can reduce the costs with increased productivity and can possess some specialized desirable characteristics. Such improved strains can be achieved by inducing genetic variation in the natural strain and subsequent screening. Thus a major effort of industrial research in producing enzymes is directed towards the screening programs. Mutation is the primary source of all genetic variation and has been used extensively in industrial improvement of enzyme production (Ghisalba et al., 1984; Sidney and Nathan, 1975). The use of mutation and selection to improve the productivity of cultures has been strongly established for over fifty years and is still recognized as a valuable tool for strain improvement of many enzyme-producing organisms.
The classical genetic approach to improve the metabolite yield is to subject the organism to random mutations using various mutagenic agents and then to screen the survivors after these lethal treatments for colonies that show increased enzyme production. This process would be repeated until no further increase could be detected. While selecting mutagen for use in strain improvement program, one should consider the phenomenon of mutagen specificity, where by a given mutagen or mutagenic treatment preferentially mutates certain parts of the genome, while unaffecting the other parts. The industrial geneticist is rarely able to predict exactly what type of mutation is required to improve the given strain. Hence a series of mutagenic treatments are carried out to develop a better yielding strain by trial and error. Various mutagenic agents such as ultraviolet rays (UV), N methyl-N’-nitro-N-nitrosoguanidine (NTG), X-rays, gamma rays, nitrous acid, ethyl methyl sulfonate (EMS) etc., are generally used for yield improvement studies.The ultra violet irradiation (UV) is the most convenient of all mutagens to use and it is also very easy to take effective safety precautions against it. The UV light is the best studied mutagenic agent in prokaryotic organisms. It gives a high proposition of pyrimidine dimers and includes all types of base pair substitutions (Meenu et al., 2000).
The EMS is also used for strain improvement as it induces linked multiple mutations at fairly high frequencies. It promotes base pair substitutions, primarily GC-AT transitions. The optimum concentration of mutagen is that which gives the highest proportion of desirable mutants in the surviving population. Hopwood et al. (1985) suggested that 99.9% kill is best suited for strain improvement programs as the fewer survivors in the treated sample would have undergone repeated or multiple mutations which may lead to the enhancement in the productivity of the metabolite. Two important conventional techniques viz., UV irradiation and EMS treatment have been carried out for yield improvement studies.
Material and Methods
Chemicals
All chemicals and medium constituents used for the present study were procured from M/S Hi-media, Mumbai. EMS was purchased from Sigma-Aldrich Chemical Co., USA.
Microorganisms
The newly isolated thermoactinomycetestrain, T. thalpophilus KS-05 (wild strain) that produce thermostable amylase, was employed in the present study. This organism was isolated from soil sample from Bidar city, Karnataka State, India. The isolate was grown on starch casein agar slants at 55oC for 24 h, subcultured at monthly intervals and stored in the refrigerator.
Preparation of spore suspension
Each organism grown on starch casein agar slant was scraped off into sterile water containing tween-80 (1:4000) to give a uniform suspension. The suspension was transferred into sterile conical flasks (250ml) containing sterile glass beads and thoroughly shaken for 30 min. on a rotary shaker to break the spore chains. The spore suspension was then filtered through a thin sterile cotton wad into a sterile tube, to eliminate vegetative cells from the suspension, so that after plating each spore was germinated to give a colony. The spore suspension was diluted and used for plating.
Shake flask fermentation
Ten ml of sterile water (2×5ml) was added to 24 h old slant of above isolates. The cells were scrapped from the slant into sterile water and from the resultant 10 ml cell suspension, 1 ml suspension was transferred aseptically into 250 ml Erlenmeyer flasks containing 50 ml each of sterile medium as mentioned above. The flasks were incubated on a rotary shaker (70 rpm) at 55°C for 24 h. The contents of the flasks were centrifuged at 3000 rpm for 10 min and the supernatant solution were tested for amylolytic activity by modified method as described Bernfield et al (1955).
Analytical Method
The amylase activity was measured in cell broths, collecting the sample at regular and predetermined incubation times according to Bernfield (1955), by estimating the maltose produced during starch hydrolysis using modified 3, 5-dinitro salicylic acid as a coupling agent. The reaction mixture containing 1.0 ml of 1% soluble starch in 0.01M Phosphate buffer (pH 7) and 1.0 ml of enzyme solution was incubated at 40oC for 5 minutes. The reaction was stopped by adding 2.0 ml of 3, 5-dinitro salicylic acid solution followed by heating in boiling water bath for 5 minutes. The contents were then cooled to room temperature and the volume was made up to 10 ml with distilled water. The absorbance of the reaction mixture was determined at 540 nm in a UV-Visible spectrophotometer. One Unit of the enzyme is defined as the amount of enzyme capable of producing 1µM of reducing sugar (as maltose) from 1% soluble starch as substrate in 1 min at 40oC.
Amylase activity (µ ml-1) = Maltose (mg)/ 360.31 × 10-6
UV irradiation of parent strains and selection of mutants
Strain improvements for the selected parent strains were done by mutation and selection. The wild strain (KS-05)was subjected to UV irradiation. The dose survival curve was plotted for selecting the mutants between 10 and 0.1% rate. Mutation frequency was mentioned to be high when the survival rates were between 10 and 0.1% (Hopwood et al., 1985). The spore suspension of wild strains was prepared in phosphate buffer, pH 7.0 and 4 ml quantities were pipetted aseptically into sterile flat-bottomed petridishes of 100 mm dia. The exposure to UV light was carried out in a “Dispensing – Cabinet” fitted with TUP 40W Germicidal lamp that has about 90% of its radiation at 2540-2550Å. The exposure was carried out at a distance of 26.5 cm away from the center of the germicidal lamp for 0, 3, 6, 9, 12, 15 and 18 min. respectively. During the exposure, the lid of the petridish was removed. Hands were covered with gloves and the plates were gently rotated so as to get uniform exposure of the contents of the petridish. During the treatment, all the other sources of light were cut off and the exposure was carried out in dark (during night time). The treated spore suspensions were transferred into sterile test tubes covered with a black paper and kept in the refrigerator over night, to avoid photo reactivation.
Each irradiated spore suspension was serially diluted with sterile phosphate buffer solution (PBS). The spore suspensions (pH 7) were plated onto starch casein agar medium and incubated for 24 h at 55°C. The number of colonies in each plate was counted. It was assumed that each colony was formed from a single spore. The number of survivals from each exposure time for isolate KS-05 is represented in Table 1.1. The UV survival curve is plotted (Fig. 1.1). Plates having less than 1% survival rate (15 and 18 min.) were selected for the isolation of mutants. The isolates were selected on the basis of macroscopic differential characteristics. The selected isolates were subjected to fermentation and tested for their amylase production capacities as described earlier (Fig. 1.2). The best amylase producing UV mutant strains (KSR-14, KSR-17 and KSR-22) were selected for EMS treatment.
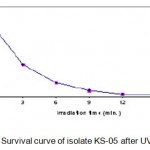 |
Figure 1.1: Survival curve of isolate KS-05 after UV irradiation.
|
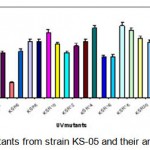 |
Figure 1.2: UV mutants from strain KS-05 and their amylase productivity.
|
Table 1.1.Effect of UV irradiation on KS-05.
| Number of cells/ml | Percentage | Survival |
| after irradiation | Kill | percent |
| 1.2 × 107 | 0 | 100 |
| 5.3 × 106 | 55.83 | 44.17 |
| 2.7 × 106 | 77.5 | 22.5 |
| 1.4 × 106 | 88.33 | 11.67 |
| 5.1 × 105 | 95.75 | 4.25 |
| 3.94 × 105 | 96.72 | 3.28 |
| 1.1 × 104 | 99.01 | 0.09 |
EMS treatment and selection of mutants
The thermoactinomycete strains (KSR-14, KSR-17 and KSR-22)were selected for EMS treatment. The cell suspensions of KSR-14, KSR-17 and KSR-22 (UV mutants of KS-05) were prepared as described earlier and the cell pellets obtained after centrifugation were resuspended in PBS (pH 7). Cell suspensions of the isolates were diluted serially to get appropriate concentrations.Five ml of each cell suspension was added to 15ml of EMS solution (4mg/ml) and incubated at 55°C (Akhund and Khvostova, 1966). Two ml of this solution was taken at intervals of 0, 30, 60, 90, 120, 150, 180, 210, and 270 min. and centrifuged immediately. The supernatant was decanted and the cell pellet obtained was resuspended in 5ml of sterile distilled water to stop the reaction. The total time from the first incubation of the cell suspension to re-suspension into distilled water was recorded. Since the cells were exposed to EMS during centrifugation period, this time has been recorded and considered as part of the total treatment time. Control was treated in the same manner without adding EMS. The washed cell suspension was serially diluted, plated on starch casein agar medium and incubated at 55°C for 24 h. After incubation, the colonies were selected on the basis of their macroscopic characteristics such as morphology, size and shape. The number of colonies in each plate was counted. Each colony was assumed to be formed from a single spore. The numbers of survivals from each exposure time for the three mutants (KSR-14, KSR-17 and KSR-22) are represented in Tables 1.2 – 1.4. The survival curves were plotted (Figs. 1.3 – 1.5).Plates having less than 1% survival rate (210 and 270 min) were selected for the isolation of mutants. The isolates were selected on the basis of macroscopic differences, subjected to fermentation and evaluated for their alkaline protease production capacities as described earlier. The results are presented in Table. 1.2.
Table 1.2: Effect of EMS on KSR-14.
| Exposure time | Number of | Percentage | Survival percent |
| (min) | cells/ml after | treatment | Kill |
| 0 | 3.6×107 | 0 | 100 |
| 30 | 3.1×107 | 13.89 | 86.11 |
| 60 | 2.8×107 | 22.22 | 65.91 |
| 90 | 5.9×106 | 83.61 | 15.45 |
| 120 | 5.3×106 | 85.28 | 13.86 |
| 150 | 6.2×105 | 98.28 | 1.8 |
| 180 | 4.1×105 | 98.86 | 1.11 |
| 210 | 3.9×105 | 98.92 | 1.01 |
| 270 | 2.7×105 | 99.25 | 0.73 |
Results and Discussion
The wild strain KS-05 was subjected to strain improvement program with a view to obtain increased amylase production and to achieve greater stability of the organism.
Isolation of UV mutants and their amylase activity
The wild strain (KS-05)was subjected to UV irradiation at varying time intervals of 0, 3, 6, 9, 12, 15 and 18 min. respectively. The number of survivals from each exposure time for isolate KS-05 is represented in Table 1.1. The UV survival curves are plotted (Fig. 1.1). Plates having less than 1% survival rate (12 and 15 min) were selected for the isolation of mutants. A total of 27 mutants were selected from parent strains KS-05 and labeled as KSR-1 to KSR-27. These mutants were tested for their alkaline protease producing capabilities as described earlier. The results are presented in Fig. 1.3 & 1.4.
Table 1.3. Effect of EMS on KSR-17.
| Exposure | Number of | Percentage | Survival percent |
| time (min) | cells/ml after | experiment | Kill |
| 0 | 2.9×107 | 0 | 100 |
| 30 | 2.6×107 | 10.34 | 89.65 |
| 60 | 1.8×106 | 37.93 | 62.07 |
| 90 | 6.1×106 | 78.97 | 21.03 |
| 120 | 5.6×106 | 80.69 | 19.31 |
| 150 | 4.8×106 | 98.34 | 1.66 |
| 180 | 4.1×106 | 98.59 | 1.41 |
| 210 | 3.6×106 | 98.76 | 1.24 |
| 240 | 2.7×105 | 99.7 | 0.93 |
Table 1.4: Effect of EMS on KSR-22.
| Exposure | Number of | Percentage | Survival percent |
| time (min) | cells/ml after | experiment | Kill |
| 00 | 3.7 × 107 | 0 | 100 |
| 30 | 3.4×107 | 8.11 | 91.89 |
| 60 | 2.9 ×106 | 21.62 | 78.38 |
| 90 | 6.4 × 106 | 82.7 | 17.3 |
| 120 | 5.1 × 106 | 86.22 | 13.78 |
| 150 | 4.2 × 106 | 88.65 | 1.35 |
| 180 | 3.6 × 106 | 99.03 | 0.97 |
| 210 | 2.9 × 105 | 99.22 | 0.78 |
| 240 | 2.1 × 105 | 99.43 | 0.57 |
The results indicated that among UV mutants, KSR-14, KSR-17 & KSR-22 from parent strain KS-05 was found to be the highest amylase producers with activities of 1924; 1986 and 1998 U/ml respectively. Hence, these mutant strains were selected for EMS treatment.
Selection of EMS mutants and their amylase production
The above selected three UV mutants (KSR14, KSR-17 and KSR-22) were subjected to EMS treatment as described above. The numbers of survivals from each exposure time for the three mutants are represented in Tables 1.3 – 1.5. The survival curves were plotted (Figs. 1.3 – 1.5).Plates having less than 1% survival rate (180 and 210 min) were selected for the isolation of mutants. A total of 21 mutants were selected, and were labeled asKSV-1 to KSV-21 and evaluated for their alkaline protease production capacities as described earlier. The results are shown in Table 1.5. The results indicated that the mutant KSV-17 (from parent KSR-22) was the highest amylase yielding strain (2476.6 U/ml).
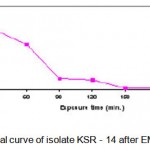 |
Figure 1.3: Survival curve of isolate KSR – 14 after EMS treatment.
|
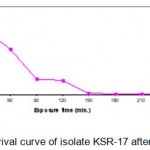 |
Figure 1.4: Survival curve of isolate KSR-17 after EMS treatment.
|
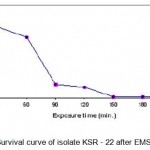 |
Figure 1.5: Survival curve of isolate KSR – 22 after EMS treatment.
|
Table 1.5.EMS mutants and their amylase activity.
| Mutants of | Mutants of | Mutants of strain | ||
| strain KSR–14 | strain KSR -17 | KSR-22 | ||
| Isolates Enzyme yield | Isolates | Enzyme yield | Isolates | Enzyme yield |
| (U/ml) | (U/ml) | (U/ml) | ||
| Control 1922.3 | Control | 1990.6 | Control | 2000.6 |
| KSV 1 2000.6 | KSV 8 | 1991.3 | KSV15 | 2012 |
| KSV 2 1433.8 | KSV 9 | 1923.5 | KSV16 | 2156.3 |
| KSV 3 1800.35 | KSV10 | 2000.6 | KSV17 | 2476.6 |
| KSV 4 2200.9 | KSV11 | 2052.8 | KSV18 | 2086.3 |
| KSV 5 1998.6 | KSV12 | 1443.3 | KSV19 | 1923.3 |
| KSV 6 900.6 | KSV13 | 1998.6 | KSV20 | 1498.3 |
| KSV 7 1736.6 | KSV14 | 2035.6 | KSV21 | 900.89 |
Thus the EMS treatment resulted in a mutant that produced 1.70 & 1.92 times higher titer of amylase over the parent strain KSR-22 (1998 U/ml), and parent wild strain KS-05 (1120 U/ml) respectively, which is a significant increase of yield. From the results, it is evident that UV and EMS were effective mutagenic agents for strain improvement of Thermoactinomyces sp.
Conclusion
We concluded that, among UV mutants, KSR-14, KSR-17 & KSR-22 from parent strain KS-05 were found to be the highest amylase producers with activities of 1924; 1986 and 1998 U/ml respectively. Hence, these mutant strains were selected for EMS treatment. After EMS treatment resulted in a mutant that produced 1.70 & 1.92 times higher titer of amylase over the parent strain KSR-22 (1998 U/ml), and parent wild strain KS-05 (1120 U/ml) respectively, which is a significant increase of yield. From the results, it is evident that UV and EMS were effective mutagenic agents for strain improvement of Thermoactinomyces
References
- Ghisalba, O., Auden, J.A.L., Schupp, T. and Nuesch, J., In: Biotechnology of Industrial Antibiotics, 3: 282 (1984).
- Sidney, P. C. and Nathan, O. K., Methods in Enzymology, 3: 26 (1975).
- Meenu, M., Santhosh, D., Kamia, C. and Randhir, S., Ind. J. Microbiol, 25: 40 (2000).
- Hopwodd, D.A., Bibb, M.J., Chater, K.F., Kieser, T., Bruton, C.J., Kieser, H.M., Lydiate, D.J., Smith, C.P., Ward, J.M. and Schrempt, H., Genetic Manipulation of Streptomyces – A Laboraroty manual, (1985).
- Bernfeld, P. Amylases,a and b , Methods in enz. Vol.I, Academic Press, New York, 149-158 (1955).
- Akhund-Zade, A.I. and Khvostova, V.V., Genetika, 2: 47 (1966).
- Shimazaki, T.; Hara, S.; Sato, M. J. Ferment. Technol, 62: 165-70 (1984).
- Pandey, A. Process Biochem,27: 109-17 (1992).
- Lonsane, B. K. and Ramesh, Adv. Appl. Biochem, 35: 1-56 (1990).
- 10 Uguru, G. C., Robb, D., Akinyanju, J. A. and Sani, A. J. Ind. Microbiol. Biotechnol, 19: 273-79 (1997).

This work is licensed under a Creative Commons Attribution 4.0 International License.





