Manuscript accepted on : 28 June 2012
Published online on: --
Shahriar Alipour1, Homayoun Khazali1 and Amir Jalili2*
1Faculty of Biological Sciences, Shahid Beheshti University, Tehran, Iran.
2Department of Biology, Islamic Azad University, Urmia branch, Urmia, Iran.
Corresponding Author E-mail: amir.jalile@yahoo.com
ABSTRACT: This study was established for determining the effects of ghrelin in the control of LH (luteinizing hormone) and T (testestrone) hormones secretion in Zell Rams fed by two different levels of their daily food requirements. Ten mature and ten immature rams were selected randomly and divided into two groups (n=5). Animals in 4 groups were fed either 100% or a 50% energy content diaet, for two weeks. Then all rams received jugular vein injection of 5 µg ghrelin/KgBW. Blood samples were withdrawn by jugular venipuncture at 30-minute intervals, 3 h before and 3 h after ghrelin injection. For determining the concentration of LH and T hormones, plasma was isolated and subjected to radioimmunoassay. The results showed that ghrelin injection in both mature and immature rams who were subjected to 50% energy content diaet, significantly reduced T hormone level. Furthermore ghrelin injection in both groups of mature rams significantly reduced LH hormone level. It seems that ghrelin have inhibitory effects on reproduction of Zell Rams by reducing sexual hormones level.
KEYWORDS: Ghrelin; Ram; reproduction; LH and T hormones
Download this article as:| Copy the following to cite this article: Alipour S, Khazali H, Jalili A. Effects of Ghrelin on LH and T Hormones secretion in mature and immature Zell Rams fed by two different levels of their daily food requirements. Biosci Biotech Res Asia 2012;9(1) |
| Copy the following to cite this URL: Alipour S, Khazali H, Jalili A. Effects of Ghrelin on LH and T Hormones secretion in mature and immature Zell Rams fed by two different levels of their daily food requirements. Biosci Biotech Res Asia 2012;9(1). Available from: https://www.biotech-asia.org/?p=9542 |
Introduction
Ghrelin is a 28-amino acid peptide that was found in the stomach and is an endogenous ligand for GHS-R (Kojima et al., 1999). It is synthesized predominantly in the stomach but has also been identified in a variety of other organs, such as bowels, kidney, thyroid, lung, lymphatic tissue, placenta, hypothalamus, and pituitary (Kluge et al., 2007). Ghrelin is the natural ligand of the GH secretagogue receptor (GHS-R) and stimulates feeding behavior and GH levels in rodents and humans. Accordingly, ghrelin stimulates GH secretion in humans and rodents (Wren et al., 2000, Javed et al., 2006). Ghrelin is secreted by a highly specialized population of cells in stomach epithelium. Secretion is tightly regulated in response to circadian rhythms, food ingestion, stress, and other factors. Plasma ghrelin concentrations rise dramatically before meals and decline immediately after eating commences (Nass et al., 2008). Its secreted into the blood stream by the endocrine stomach mucosal cells named “X/A like” in rat (Rindi et al., 2002, Perdona et al., 2011) and P/D1 cells in humans (Sakata et al., 2002). Once released, ghrelin reaches the pituitary, where it binds to its receptor and stimulates the secretion of growth hormone. Ghrelin receptors are also located in several other central nervous system sites, including many known to be involved with body weight determination (Zigman et al., 2006). Administration of excess ghrelin leads to increased food intake in rodents and humans. Therefore, ghrelin has been considered to be an important factor in regulating food intake (Kojima et al., 2006, Cummings et al., 2006). Ghrelin has been implicated in the regulation of other endocrine functions, with evidence of effects in the rat, human, and monkeys on the secretion of prolactin (PRL), adrenocorticotrophic hormone, cortisol (Tassone et al., 2003, Broglio et al., 2004, Javed et al., 2006), and LH (Fernandez et al., 2004, Vulliemoz et al 2004). Whereas leptin (Ahima and Osei, 2004), insulin (Plum et al, 2005), and other peripheral factors act as satiety factors (Park and Bloom, 2005). Although ghrelin is not essential for food intake in mice, it is required for certain food reward behaviors that occur in the setting of chronic calorie restriction (Perello et al., 2010). Ghrelin is also essential in mice to prevent hypoglycemia and death when the animals are subjected to severe calorie restriction. The latter function of ghrelin became apparent in studies of genetically engineered mice that lack the gene encoding ghrelin O-acyltransferase (GOAT), the enzyme that attaches octanoate to ghrelin, a reaction that is essential for the biological activity of the hormone (Zhao et al., 2010). Administration of ghrelin decreases LH and T in human and animal models (Barreiro and Sempere 2004, Kluge et al. 2007, Garcia et al. 2007). There are few studies about the effect of ghrelin on reproduction activity axis in different strains of ruminants under different level of energy. So, the goal of this study was to determine the effect of ghrelin on LH and T secretions in the mature and immature Zell rams fed by two different levels of their daily food requirements.
Materials and Methods
Ten mature (Average Weight= 57±2.3 kg) and ten immature (Average Weight= 32±1.4 kg) rams were randomly selected from Rasht Research Center for Agriculture and Natural Resources. Animals in four groups (n=5) were fed respectively either by 50% or 100% of their daily food requirement for two weeks. After two weeks, rams received 5 µg ghrelin/kg body weight via their jugular vein. Total energy and chemical compositions of feedstuffs, namely, crude fiber, crude protein, fresh and dry matter, NDF, ADF, ether extract, ash, calcium, and phosphorous, were analyzed in the Rasht Research Center for Agriculture and Natural Resources. Animals were maintained on the diets for two weeks. Diets were formulated based on maintenance energy requirements and according to standard tables (Ensminger and Parker, 1986). Blood samples were collected by jugular vein at 30-min intervals (Mcmanus et al., 2000), 3 h before and 3 h following galanin injection. The samples were then centrifuged for 15 minutes and the plasma was stored at -20°C until assayed for LH and T levels. Blood samples that were collected before the injection of ghrelin in the four treatments served as the controls for each group. Plasma concentrations of LH and T were determined by double-antibody radioimmunoassay (RIA) with standard LH and T ovine antigens (Tabeshyarnoor Co., Iran). ANOVA was used to assess any statistical differences in mean LH and T levels in animals in response to ghrelin. All statistical analyses were performed using SPSS (version 13). The results are shown as means ± SE and the level of significance was set at p < 0.05.
Results and discussion
LH
Mean LH plasma levels were significantly (P_0.05) decreased in both groups of mature rams fed by 100% (M-F) or 50% (M-NF) of their daily food requirement, after the Administration of Ghrelin (Figure 1). Since mean LH plasma levels were not affected significantly in immature rams (Figure 2).
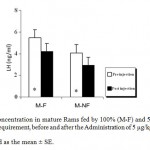 |
Figure 1: LH Concentration in mature Rams fed by 100% (M-F) and 50% (M-NF) of their daily food requirement, before and after the Administration of 5 μg/kg BW Ghrelin.
|
* p < 0.05
Data is presented as the mean ± SE.
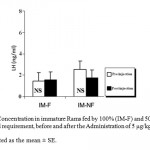 |
Figure 2: LH Concentration in immature Rams fed by 100% (IM-F) and 50% (IM-NF) of their daily food requirement, before and after the Administration of 5 μg/kg BW Ghrelin.
|
Data is presented as the mean ± SE.
T
The ANOVA analysis also revealed that the mean T plasma levels were significantly (P_0.05) decreased in both groups of mature and immature rams fed by 50% (M-NF) of their daily food requirement, after the Administration of Ghrelin (Figure 3). Since its concentration were not affected significantly in mature and immature rams fed by 100% (M-F) of their daily food requirement (Figure 4).
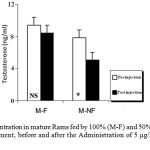 |
Figure 3: T Concentration in mature Rams fed by 100% (M-F) and 50% (M-NF) of their daily food requirement, before and after the Administration of 5 μg/kg BW Ghrelin.
|
* p < 0.05
Data is presented as the mean ± SE.
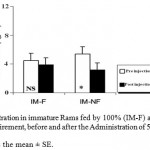 |
Figure 4: T Concentration in immature Rams fed by 100% (IM-F) and 50% (IM-NF) of their daily food requirement, before and after the Administration of 5 μg/kg BW Ghrelin.
|
* p < 0.05
Data is presented as the mean ± SE.
The data of the present study indicate that ghrelin decreases LH and T Concentration especially in mature Zell rams. Similar results were reported on human (Unniappan, 2009), rodents (Javed et al., 2006), monkey (Vulliémoz et al., 2004) and rats (Fernandez et al., 2004). There is only one study in humans, which indicates that Ghrelin has no effect on LH (Takaya et al., 2000). In rat gonadotropes, two distinct subsets of secretory granules have been identified (small dense granules rich in LH and large lucent granules rich in FSH) (Farifteh et al., 2008). Ghrelin was found to affect the hypothalamic-pituitary-gonadal axis at all levels. However, only for the hypothalamic level has evidence been provided from both in vitro and in vivo experiments (Tenasempere, 2005). It is possible that ghrelin differentially modulates the intracellular actions of LHRH (Fernandez et al., 2004), a finding previously described for peptides involved in the control of LHRH action, such as endothelins (Kauyicska et al., 1991), galanin (Aboutalebi et al., 2011) and NPY (Evans, 1999). Ghrelin significantly decreased hypothalamic LHRH in vitro (Fernandez et al., 2006). In addition, a decrease of the LH pulse frequency in vivo, as found in humans (Ogata et al.,2009) and animals (Furuta et al., 2001, Vulliemoz et al., 2004), indicates an inhibition of the hypothalamic LHRH pulse generator. This inhibitory effect was suggested to be mediated by hypothalamic neuropeptide Y and agouti-related peptide (Vulliemoz et al., 2004) because both peptides have inhibited pulsatile LH release (Shahab et al., 2003), and their synthesis has been decreased by ghrelin (Goto et al., 2006). Interestingly, not only acylated ghrelin but also the unacylated isoform of ghrelin, which has been considered widely inert (VanderLely et al., 2004), decreased LH secretion in rats to a similar extent as the acylated isoform. This finding indicates that central effects of ghrelin on the hypothalamic-pituitary-gonadal axis are not, or at least not only, mediated through the GHS-R that the unacylated form does not bind to (Martini et al., 2006).
To the best of our knowledge, this is the first study in which the effects of ghrelin on LH and T secretion in rams fed by two different energy levels have been investigated. The results of this study suggest that the effect of ghrelin on LH and T secretion in rams is independent from the metabolic state and energy balance of the animal. Most probably, the decrease of energy in the diet can not change the sensitivity of hormones secretion toward ghrelin. In conclusion, ghrelin is a peptide hormone mainly secreted from the stomach into the circulation, but it can be synthesized by other tissues such as reproductive tissues suggesting local actions (autocrine and/or paracrine). Ghrelin participates in the regulation of different aspects of the reproductive functions of rams. Ghrelin through its extensive biological functions including energy metabolism by promoting fat deposition and food intake could be a key signal between energy status and control of fertility. However, further studies are required to gain insights into the understanding of the fine mechanisms of ghrelin action.
References
- Aboutalebi F, Khazali H, Emami MA (2011). Effects of high energy intact and treatment with galanin on gonadotropin levels in femaile goats. Int. J. Endocrinol. Metab. 9(2):271-275.
- Ahima RS, Osei SY (2004). Leptin signaling. Physiol. Behav. 81:223–241.
- Barreiro ML, Sempere TM (2004). Ghrelin and reproduction: a novel signal linking energy status and fertility. Mol. Cell. Endocrinol. 226(1-2):1-9.
- Broglio F, Gottero C, Prodam F, Gauna C, Muccioli G, Papotti M, Abribat T, Vanderely AJ, Ghigo E (2004). Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. J. Clin. Endocrinol. Metab. 89:3062–3065.
- Cummings DE (2006). Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol. Behav. 89: 71–84.
- Ensminger ME, Parker RO (1986). Sheep and goat science. 5th ed. Danville, Illinois, USA: Interstate Printers and Pub.
- Farifteh F, Khazali H, Emami M (2008). Effect of Ghrelin on Plasma Concentration of Gonadotropins in the Female Sannan Goats. Int. J. Endocrinol. Metab. 1: 28-33.
- Fernandez-Fernandez R, Tena-Sempere M, Aguilar E, Pinilla L (2004). Ghrelin effects on gonadotropin secretion in male and female rats. Neurosci. Lett. 362:103–107.
- Fernandez-Fernandez R, Tena-Sempere M, Navarro VM, Barreiro ML, Castellano JM, Aguilar E, Pinilla L (2006). Effects of ghrelin upon gonadotropinreleasing hormone and gonadotropin secretion in adult female rats: in vivo and in vitro studies. Neuroendocrinology. 82:245–255.
- Garcia MC, Lopez M, Alvarez CV, Casanueva F, Tena-Sempere M, Dieguez C (2007). Role of ghrelin in reproduction. Reproduction. 133(3): 531-540.
- Goto M, Arima H, Watanabe M, Hayashi M, Banno R, Sato I, Nagasaki H, Oiso Y (2006). Ghrelin increases neuropeptide Y and agouti-related peptide gene expression in the arcuate nucleus in rat hypothalamic organotypic cultures. Endocrinology. 147:5102–5109.
- Furuta M, Funabashi T, Kimura F (2001). Intracerebroventricular administration of ghrelin rapidly suppresses pulsatile luteinizing hormone secretion in ovariectomized rats. Biochem. Biophys. Res. Commun. 288:780–785.
- Iqbal J, Kurose Y, Canny B, Clarke IJ (2006). Effects of Central Infusion of Ghrelin on Food Intake and Plasma Levels of Growth Hormone, Luteinizing Hormone, Prolactin, and Cortisol Secretion in Sheep. Endocrinology. 147(1):510–519.
- Kluge M, Schussler P, Uhr M, Yassouridis A, Steiger A. (2007). Ghrelin suppresses secretion of luteinizing hormone in humans. J. Clin. Endocrinol. Metab. 92(8):3202-5.
- Kluge M, Schussler P, Zuber V, Yassouridis A, Steiger A (2007). Ghrelin administered in the early morning increases secretion of cortisol and growth hormone without affecting sleep. Psychoneuroendocrinology. 32:287–292.
- Kojima M, Kangawa K (2005). Ghrelin: Structure and function. Physiol. Rev. 85:495–522.
- Martini AC, Fernandez-Fernandez R, Tovar S, Navarro VM, Vigo E, Vazquez MJ, Davies JS, Thompson NM, Aguilar E, Pinilla L, Wells T, Dieguez C, Tena-Sempere M (2006). Comparative analysis of the effects of ghrelin and unacylated ghrelin on luteinizing hormone secretion in male rats. Endocrinology. 147:2374–2382.
- Mcmanus CJ, Fitzgerald BP (2000). Effects of a single day of feed restriction on changes in serum leptin, gonadotropins, prolactin, and metabolites in aged and young mares. Domest. Anim. Endocrinol. 19(1):1-13.
- Nass R, Farhy LS, Liu J, Prudom CE, Johnson ML, Veldhuis P, Pezzoli SS, Oliveri MC, Gaylinn BD, Geysen HM, Thorner MO. (2008) Evidence for acyl-ghrelin modulation of growth hormone release in the fed state. J. Clin. Endocrinol. Metab. 93:1988–1994.
- Ogata R, Matsuzaki T, Iwasa T, Kiyokawa M, Tanaka N, Kuwahara A, Yasui T, Irahara M (2009). Hypothalamic Ghrelin suppresses pulsatile secretion of Luteinizing hormone via -endorphin in ovariectomized rats. Neuroendocrinol. 90: 364-370.
- Park AJ, Bloom SR (2005). Neuroendocrine control of food intake. Curr. Opin. Gastroenterol. 21:228–233.
- Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, Woloszyn J, Yanagisawa M, Lutter M, Zigman JM. (2010). Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol. Psychiatry. 67:880–886.
- Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR (2000). The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 141:4325–4328.
- Rindi G, Savio A, Torsello A, Zoli M, Locatelli V, Cocchi D, Paolotti D, Solcia E (2002). Characterisation of gastric ghrelin cells in man and other mammals: studies in adult and fetal tissues. Histochem. Cell. Biol. 117(6):511–519.
- Perdona E, Faggioni F, Buson A, Sabbatini FM, Corti C, Corsi M (2011). Pharmacological characterization of the ghrelin receptor antagonist, GSK1614343 in rat RC-4B/C cells natively expressing GHS type 1a receptors. European. J. Pharmacol. 650(1):178–183.
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K (1999). Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 402:656–660.
- Sakata I, Nakamura K, Yamazaki M, Matsubara M, Hayashi Y, Kangawa K, Sakai T (2002). Ghrelinproducing cells exist as two types of cells, closed- and openedtype cells, in the rat gastrointestinal tract. Peptides. 23(3): 531–536.
- Shahab M, Balasubramaniam A, Sahu A, Plant TM (2003). Central nervous system receptors involved in mediating the inhibitory action of neuropeptide Y on luteinizing hormone secretion in the male rhesus monkey (Macaca mulatta). J. Neuroendocrinol. 15:965–970.
- Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M. (2000). Ghrelin strongly stimulates growth hormone release in humans. J. Clin. Endocrinol. Metab. 85: 4908-11.
- Tassone F, Broglio F, Destefanis S, Rovere S, Benso A, Gottero C, Prodam F, Rossetto R, Gauna C, van der Lely AJ, Ghigo E, Maccario M (2003). Neuroendocrine and metabolic effects of acute ghrelin administration in human obesity. J. Clin. Endocrinol. Metab. 88:5478–5483.
- Tena-Sempere M (2005). Ghrelin: novel regulator of gonadal function. J. Endocrinol. Invest. 28:26–29.
- Unniappan S (2009). Ghrelin: An emerging player in the regulation of reproduction in non-mammalian vertebrates. Gen. Comp. Endocrinol. 167(3): 340-3.
- Vanderlely AJ, Tschop M, Heiman ML, Ghigo E (2004). Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr. Rev. 25:426–457.
- Vulliemoz NR, Xiao E, Xia-Zhang L, Germond M, Rivier J, Ferin M (2004). Decrease in luteinizing hormone pulse frequency during a five-hour peripheral ghrelin infusion in the ovariectomized rhesus monkey. J. Clin. Endocrinol. Metab. 89:5718–5723.
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR (2001). Ghrelin enhance appetite and increase food intake in humans. J. Clin. Endocrinal. Metab. 86:5992-5995.
- Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Goldstein JL, Brown MS (2010). Ghrelin O-acyltransferase (GOAT) is essential for growth hormonemediated survival of calorie-restricted mice. Proc. Natl. Acad. Sci. 107:7467–7472.
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK (2006). Expression of ghrelin receptor mRNA in the rat and the mouse brain. J. Comp. Neurol. 494:528–548.

This work is licensed under a Creative Commons Attribution 4.0 International License.





