How to Cite | Publication History | PlumX Article Matrix
D. Sengupta, S. Ganguly* and A.K. Banik
Department of Chemical Engineering Biochemical Engineering Division, Biotechnology Laboratory, University of Calcutta, 92, A. P. C. Road, Kolkata - 700 009 India.
Corresponding Author E-mail: subhadeepgangulyphysiol@rediffmail.com
ABSTRACT: Thiobacillus ferrooxidans treated with ethylmethane sulfonate and UV rays was incubated with coal sample taken from Assam, India for its desulfurization capacity. 2% glucose and 0.7% NH4Cl served as the best carbon and nitrogen sources respectively. Optimum concentration of both MgCl2 and KH2PO4 in fermentation medium was 0.025% supplementation of medium with 10 µg/ml Fe2(SO4)3.H2O had marked positive effect on desulfurization. Optimization of chemical nutrients resulted in a significant (p<0.05) impact on desulfurization (66.1%) by the mutant strain Thiobacillus ferrooxidans X200 as against parent strain (55.1%).
KEYWORDS: Thiobacillus ferrooxidans; Desulfurization; Optimization; Chemical nutrients
Download this article as:| Copy the following to cite this article: Sengupta D, Ganguly S, Banik A. K. Stimulatory Effect of Chemical Nutrients on Desulfurization of Indial Coal by a Mutant Thiobacillus ferrooxidans X200. Biosci Biotech Res Asia 2011;8(2) |
| Copy the following to cite this URL: Sengupta D, Ganguly S, Banik A. K. Stimulatory Effect of Chemical Nutrients on Desulfurization of Indial Coal by a Mutant Thiobacillus ferrooxidans X200. Biosci Biotech Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9548/ |
Introduction
Coal deposits of North-Eastern India have a high sulfur content (2-6%)1. During the combustion of these sulfur – bearing coal emissions of volatile sulfur compounds like SO2, SO3 and H2S cause air pollution as well as corrosion in boilers2. These problems need an efficient and economic method of coal desulfurization at its source. Precombustion removal of sulfur from coal by microbial action presents an alteractive alternative to the existing physical or chemical methods because it is cost – effective and runs under ambient temperature and pressure conditions. Microorganisms frequently used for this purpose are Thiobacillus ferrooxidans, Thiobacillus thiooxidans, Thiobacillus acidophilus, Bacillus brevis etc.3-17.
The organism used in our study for desulfurization of coal is Thiobacillus ferrooxidans which may be described as an iron and sulfur oxidizing bacterium that catalyzes the removal of inorganic (mainly pyritic) sulfur from Coal3. We have already developed a mutant strain of Thiobacillus ferrooxidans by using multistep mutagenic agents like ethyl methane sulfonate an UV-irraditions. The mutant Strain Thiobacillus ferrooxidans X200 has a higher sulfur removing capacity (32%) than the parsent strain (10%). The optimum conditions of Physical parameters for desulfurization of coal by Thiobacillus ferrooxidans X200 have been also developed earlier4.
The present study deals with the nutritional requirements of the bacterium, viz. concentrations of the suitable carbon source, nitrogen source, MgCl2 and KH2PO4. The effect of supplementation of the medium by Fe2(SO4)3, H2O was also studied.
Materials and Methods
Source and composition of coal
Coal samples used in this experiment were obtained from North Eastern Coal fields, Coal India Ltd., Margherita, Assam. The sample was found to contain 3.03% total sulfur in which 14.7% was pyritic sulfur, 20.5% was sulfate sulfur an 64.7% was organic sulfur.
Microorganism
The present strain of Thiobacillus ferrooxidans was obtained from the Department of Molecular Biology, Biophysics and Genetic Engineering, University of Calcutta. The culture was maintained in FeSO4 medium which was a 7 : 3 (v/v) mixture of two media of following composition : Medium A – (NH4)2SO4, 0.43%; MgSO4.7H2O, 0.07%; KH,PO4, 0.07%; KCl, 0.17; 10(N)H2SO4, 0.03 ml in 100 ml deionized double distilled water. Medium B – FeSO4.7H2O, 10.2%; 10(N) H2SO4, 0.2 ml in 100 ml deionized double distilled water. The pH of the medium was adjusted to 4.0. The parent strain on exposure to ethyl methane sulfonate [0.2 (M) for 120 mins] and UV rays 15 watt Hanovia germicidal lamp for 30 mins) gave the mutant strain Thiobacillus ferrooxidans X200 which had a higher sulfure removing capacity (32%) from coal.
Fermentation medium and cultural conditions
The fermentation medium for desulfurization of coal by Thiobacillus ferrooxidans X200. The fermentation was carried out for 8 days.
Determination of optimum concentration of suitable chemical nutrients
The suitable carbon and nitrogen source for Thiobacillus ferrooxidans X200 was selected by substituting glucose and NH4Cl with equivalent amount of different carbon and nitrogen sources respectively. The optimum concentration of the suitable carbon and nitrogen source was determined by performing fermentation experiments with different concentrations of most the most effective carbon and nitrogen source. The optimum concentrations of MgCl2 and KH2PO4 were also determined by conducting experiments with different concentrations of MgCl2 and KH2PO4 respectively. To study the effect of Fe2(SO4)3.H2O all the media components were made free from mineral impurities by the process of solvent extraction where chloroform was used as the solvent and 8-hydroxyquinole as the chelating agent8. Thereafter, fermentation experiments were performed with different concentrations of Fe2(SO4)3.H2O.
Estimation of sulfur content in coal
Total sulfur of coal was determined by the conventional Eschka’s method18. Pyritic sulfur and sulfate sulfur was also estimated according to the standard procedures10. The amount of organic sulfur on coal was obtained by subtracting the amount of pyritic sulfur and sulfate sulfur from the total sulfur content. The treated coal samples were futered, dried and analysed for total sulfur content.
Estimation of pH
pH of the fermentation broth was estimated by electronic pH meter.
Statistical analysis
Values were expressed as mean ± SEM, where n=6. The data were analysed using one way ANOVA followed by Dunett’s post hoc multiple comparison test using “prism 4.0” soft ware (Graph pad Inc., USA). A “p” value less than 0.05 was considered significan and less than 0.01 was considered highly significant.
Result and Discussion
Effect of different carbon sources
Carbon is required by the bacterium for cellular growth6. Carbon dioxide serves as the major source of cell carbon for growing Thiobacillus ferrooxidans X200. Carbonate minerals are also used a substitute corbon source2. But addition of CaCO3 may inhibit the bacteria to oxidize pyrite due to the acid nutrilizing effect of the carbonate mineral which raise the pH of the leach solution above the upper limit of bacterial activity. Studies with different carbon sources revealed that glucose was the best utilizable carbon (Fig.1).
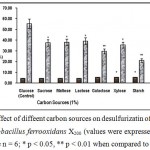 |
Figure 1 : Effect of diffeent carbon sources on desulfurizatin of Coal by the mutant Thiobacillus ferrooxidans X200 (values were expressed as Mean ± SEM, where n = 6; * p < 0.05, ** p < 0.01 when compared to control). |
Determination of optimum concentraton of glucose
The dependence of desulfurization capacity of Thiobacillus ferrooxidans X200 on glucose concentration is depicted in Fig.2 which shows that the optimum glucose concentration is 2%. Retardation of desulfurization process at higher glucose concentration may be explained by increased substrate concentration above the critical value.
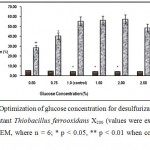 |
Figure 2 : Optimization of glucose concentration for desulfurizatin of Coal by the mutant Thiobacillus ferrooxidans X200 (values were expressed as Mean ± SEM, where n = 6; * p < 0.05, ** p < 0.01 when compared to control). |
Effect of different nitrogen sources
Nitrogen, as NH4+ ion, is essential for bacterial growth7. The nitrogen requirement of Thiobacillus ferrooxidans X200 was examined using different nitrogen sources (Fig.3). Most f the ionic salts proved out to be good nitrogen source for Thiobacillus ferrooxidans X200 among which NH4Cl is most effective. Urea and ammonium acetate proved out to be less effective as nitrogen source8.
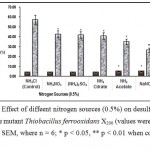 |
Figure3 : Effect of diffeent nitrogen sources (0.5%) on desulfurizatin of Coal by the mutant Thiobacillus ferrooxidans X200 (values were expressed as Mean ± SEM, where n = 6; * p < 0.05, ** p < 0.01 when compared to control). |
Determination of optimum concentrations of NH4Cl
Fig.4 shows that maximum desulfurization of coal by Thiobacillus ferrooxidans X200 has occurred when the concentration of NH4Cl is 0.07%. Desulfurization capacity of the bacteria decreased in both lower (0.04%) and higher (0.15%) range of concentrations.
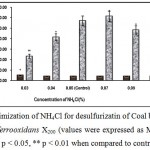 |
Figure 4 : Optimization of NH4Cl for desulfurizatin of Coal by the mutant Thiobacillus ferrooxidans X200 (values were expressed as Mean ± SEM, where n = 6; * p < 0.05, ** p < 0.01 when compared to control). |
Te composition of the fermentations medium after optimization of the chemical nutrients is glucose, 2%; NH4Cl, 0.07%; MgCl2, 0.025%; KH2PO4, 0.025%; Fe2(SO4)3.H2O 10 µg/ml and yeast extract, 0.05%. Further studies are in progress to investigate the effect of other metal ions and complex nutrients on the desulfurization of coal by Thiobacillus ferrooxidans X200.
Determination of optimum concentrations of MgCl2 and KH2PO4 :
Magnesium, phosphorus, potassium etc. are essential for bacterial growth and most growth media for Thiobacillus sp. contain these substances in various quantities. Mg++ was added to the fermentation medium for Thiobacillus ferrooxidans X200 as MgCl2. The effect of different concentrations of MgCl2 is given in Fig.5, which shows that optimum concentration for MgCl2 is 0.025%.
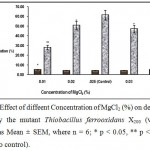 |
Figure 5 : Effect of diffeent Concentration of MgCl2 (%) on desulfurizatin of Coal by the mutant Thiobacillus ferrooxidans X200 (values were expressed as Mean ± SEM, where n = 6; * p < 0.05, ** p < 0.01 when compared to control). |
KH2PO4 was used as the source for both K+ ion and phosphorus in the leaching medium. Fig.6 shows that optimum concentration of KH2PO4 is 0.025%. Higher phosphate concentration inhibited the desulfurization process by formation of insoluble iron phosphates with the pyritic particles of coal8.
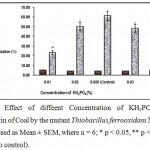 |
Figure 6 : Effect of diffeent Concentration of KH2PO4 (%) on desulfurizatin of Coal by the mutant Thiobacillus ferrooxidans X200 (values were expressed as Mean ± SEM, where n = 6; * p < 0.05, ** p < 0.01 when compared to control). |
The precipitation of the complex was found to be minimum at the optimum N/P ratio of 7.07 where NH4+ ion counteract the apparent phosphate inhibition.
Effect of different concentration of Fe2(SO4)3.H2O
Supplementation of fermentation medium for desulfurization of coal by Thiobacillus ferrooxidans had the general effect of increasing the removal of pyrite as Fe2(SO4)3 could oxidize pyrite chemically”. But high concentration of Fe+++ might lead to the deposition of insoluble complexes on the coal surface. Consequently there is an optimal relation between ferric ion concentration and bioleaching ratee12. Fig.7 shows that the optimal concentration of Fe2(SO4)3.H2O is 10 µg/ml and addition of Fe+++ in excess of the optimal concentration decrease bioleaching rate.
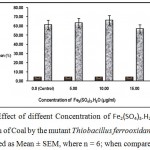 |
Figure 7 : Effect of diffeent Concentration of Fe2(SO4)3.H2O (µg/ml) on desulfurizatin of Coal by the mutant Thiobacillus ferrooxidans X200 (values were expressed as Mean ± SEM, where n = 6; when compared to control). |
Reference
- Jeszczak A., Domka F., Kozlowski M and Wachowska H., Fuel., 74(5), 725 (1995).
- Pichtel J. R. and Dick W. A., Soil Biol. Biochem., 23(2), 101 (1991).
- Silverman M. P. and Lundgren D. G., J. Bacteriol., 17. 642 (1959).
- Silverman M. P., Rogoff M. H. and Wendes , Fuel., 42, 113(1963).
- Indian Standard, I S : 1350 (Part III), 5-3(1969).
- Brierley C. L., CRC, Crit. Rev. Microbiol., 6, 207(1978).
- Hoffmann M. R., Faust B. C., Panda F. A., Koo H. H. and Tsuchiya H. M., Appl. Environ. Microbiol., 42(2), 259 (1981).
- Olsen G. J. and Brinckman F. E., Fuel., 65, 1638 (1986).
- Eligwe C. A., Fuel., 67, 451 (1988).
- Komishi Y., Kubo H. and Asai S., Biotechnol. Bioeng., 39(1), 66 (1992).
- Konishi Y., Kawamura T. and Asai S., J. Chem Japan, 26(1), 83 (1993).
- Shennan J. L., J. Chem. Tech. Biotechnol., 67, 109-123 (1996).
- Monticelo D. J., CEMTECH., 28(7), 38-45 (1998).
- Sengupta D., Das S. K., Banik A. K., Int. Conf. on Disperse Systems, 19-21 (1998).
- Ohshiro T. and Izumi Y., Biosci. Biotechnol : Biochem., 63(1), 1-9(1999).
- Olson, S. Patent 6124130(2000).
- Xiong X. C., Li W. L., Li X., Xing J. M. and Liu H. Z., Wei Sheng Wu Xue Bao., 45(5), 733-737 (2005).
- Ahmed S. M. and Whalley B. J. P., Fuel., 51(3), 190-193(1972).

This work is licensed under a Creative Commons Attribution 4.0 International License.





