How to Cite | Publication History | PlumX Article Matrix
V. Sridevi1, M. V. V. Chandana Lakshmi1, A. V. N. Swamy2, M. Narasimha Rao3 and S. V. Naidu3
1Centre for Biotechnology, Department of Chemical Engineering, Andhra University, Visakhapatnam - 03 India. 2Department of Chemical Engineering, College of Engineering, J.N.T.U., Anantapur India. 3Al-Ameer College of Engineering and Information Technology, College of Engineering, Gudilova, Anadapuram, Visakhapatnam India. Corresponding Author E-mail:vellurusridevi@yahoo.co.in
ABSTRACT: Phenol biodegradation by Pseudomonas fluorescence (NCIM 2100) was performed in batch system. Experiments were carried out as a function of inoculum size (1-10 % v/v), p H (5-9) and temperature (27-32°C). Optimization of these three process parameters for phenol biodegradation was studied. Optimum inoculum size, p H and temperature were determined as 5% v/v, 7 and 30°C respectively. At these optimum conditions, the degradation capability of P. fluorescence was carried out at phenol levels (100- 750 mg/l). The maximum phenol degradation of P. fluorescence was 480 mg/l.
KEYWORDS: Biodegradation; phenol; Pseudomonas fluorescence; inoculum size; pH; temperature.
Download this article as:| Copy the following to cite this article: Sridevi V, Lakshmi M. V. V. C, Swamy A. V. N, Rao M. N, Naidu S. V. Optimization of Process Variables for the Microbial Degradation of Phenol by Pseudomonas Fluorescence (NCIM 2100). Biosci Biotech Res Asia 2010;7(1) |
| Copy the following to cite this URL: Sridevi V, Lakshmi M. V. V. C, Swamy A. V. N, Rao M. N, Naidu S. V. Optimization of Process Variables for the Microbial Degradation of Phenol by Pseudomonas Fluorescence (NCIM 2100). Biosci Biotech Res Asia 2010;7(1). Available from: https://www.biotech-asia.org/?p=9574 |
Introduction
The environment, as a consequence of industrial and agricultural revolutions, has been hardened with potentially carcinogenic and mutagenic halogen-substituted aromatic compounds1-3. Among which phenol is a listed priority pollutant by the U.S. Environmental Protection Agency4 and is consider to be a toxic compound by the Agency for Toxic substances and Disease Registry5. Phenol and its derivatives which represent major organic pollutant in the effluents, from many industrial activities such as oil refineries, chemical, petrochemical, pharmaceutical, metallurgical, pesticide product, paint and varnish industries, textile and also in the polymer industries, like phenolic resins, bisphenol A, alkylphenols, caprolactums and adipic acid6. Phenol is a toxic and hazardous substance even at low concentrations7, 8. The concentration of phenols in wastewaters varies from 10 to 300 mg/l 6. The adverse effects of phenol on health are well documented9 and death among adults has been reported with ingestion of phenol ranging from 1 to 32 g10. The low volatility of phenol and its affinity for water make oral consumption of contaminated water, the greatest risk to humans10.
Efficient treatment methods are necessary to reduce phenol concentration in wastewater to acceptable level, which is 5 ppm (USEPA). Several methods available for treatment of phenol in which bioremediation is receiving the most attention due to its environmentally friendly, its ability to completely mineralize toxic organic compounds without producing innocuous end products and minimum secondary waste generation11 and of low-cost12, 10 . Microbial degradation of phenol has been actively studied and these studies have shown that phenol can be aerobically degraded by wide variety of fungi and bacterial cultures such as Acinetobacter species W-17 13,14 , Bacillus brevis15, 16; Microbacterium phyllospaerae17; Pseudomonas cepacia18; Pseudomonas putida CCRC 1436519; Pseudomonas aeruginosa20; Pseudomonas fluorescence20; Pseudomonas desmolyticum21 ; Fusarium22,23 ; Candida tropicalis24-26 and Ankistrodesmus braunii27.
It has been demonstrated that treatment of small volumes of toxic compounds at the point of emission using specific microbial strains and better bioreactors allows a higher control over the process and higher removal efficiencies28 .Thus optimization of process variables is recognized to be an essential aspect of successful fermentation29.
In order to find a strain, able to degrade phenol in a changeable environment, we studied the influence of inoculum size, effect of pH, and effect of temperature on phenol degradation by P. fluorescence .Phenol degradation was also studied with different level of phenol concentrations (100-750 mg/l) at these optimized conditions.
Materials and Methods
Chemicals
Phenol (99% pure, chemical grade) 4-amino antipyrine and all other chemicals used were from Merck.
Source of organism
The microorganism P. fluorescence was obtained from culture collection (NCL) Pune, India and it was maintained on a medium containing Beef extract: 1.0 g/l, Yeast extract: 2.0 g/l, Peptone: 5.0 g/l, NaCl: 5.0 g/l and Agar: 20 g/l. The pH of the medium was adjusted to 7.0 by adding 1N NaOH. It was stored at 300C for further use.
Growth determination
To study the extent of degradation, the cells were grown in a Minimal Salt (MS) medium with the following composition: Phenol 0.100 g/l; K2HPO4, 1.5 g/l; KH2PO4, 0.5 g/l; (NH4)2SO4, 0.5 g/l; NaCl, 0.5 g/l; Na2SO4, 3.0 g/l; Yeast extract, 2.0 g/l; Ferrous sulfate, 0.002 g/l; CaCl2, 0.002 g/l in conical flask containing and inoculated with P. fluorescence. The experimental studies were carried out in shake flasks with agitation at a rate of 120 rpm, temperature at 300C. Bacterial growth was determined in terms of cell mass by measuring optical density at a wavelength of 500nm.
Effect of inoculum size on phenol degradation
The effect of inoculum size (1 – 10% v/v) on phenol degradation was tested. Cells were grown as shake cultures at 300C in MS medium supplemented with 100 mg/l phenol at pH 7 for P. fluorescence in 250 ml Erlenmeyer flasks. At different times, growth and phenol degradation were measured.
Effect of pH of the medium on phenol degradation
fluorescence with an optimum inoculum size, (5%v/v) was grown in MS medium with 100 mg/l of phenol at different pH values 5 to 9.This mixture was contained in 250 ml Erlenmeyer flasks. The cultures were incubated in an orbital shaker at 300C with agitation speed 120 rpm. At different times, growth and phenol degradation were measured.
Effect of temperature of the medium on phenol degradation
fluorescence was grown in MS medium with 100 mg/l of phenol at different temperatures (27°C, 280C, 290C, 300C and 320C), inoculum size 5% v/v and pH 7 in 250 ml Erlenmeyer flasks. The cultures were placed on a shaker (120 rpm) at the above mentioned temperatures. At different times, growth and phenol degradation were measured.
Estimation of phenol
Phenol was determined quantitatively by the Spectrophotometric method (DR/ 4000 V, Hach) using 4-amino antipyrine as the color reagent (λ max: 500nm) according to standard methods of analysis30.
Growth determination
Bacterial growth was determined in terms of cell mass by measuring optical density at a wavelength of 500nm.
Results and Discussion
Effect of amount of inocula on phenol degradation
Effect of various amounts of inocula 1- 10 %v/v was studied on phenol degradation with a level of phenol concentration, 100 mg/l. Hill and Robinson,1975 concluded the size of inoculum might affect the duration of the lag phase. The data in the Fig.-1 (all the data not shown for clarity) show that phenol was almost completely reduced after incubation for 120 hrs in the presence of different concentrations of incoula in the medium. Whereas the concentration of phenol declined quickly when more cells were inoculated. Phenol degradation which was influenced by the amount of inocula, 5%v/v of biomass concentration provided the best degradation in short duration.
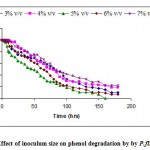 |
Figure 1: Effect of inoculum size on phenol degradation by by P.fluorescence.
|
Effect of pH of the medium on phenol degradation
The hydrogen ion concentration in the culture medium greatly influences the bacterial growth since p H limits the activity of enzymes. Variations in the p H of the medium resulted in changes in the ionic form of the active site and changes in the activity of the enzymes and hence the biodegradation rate. Changes in p H may also alter the three dimensional shape of the enzymes in microorganisms. For these reasons enzymes in microorganisms are only active over a certain p H range and different microorganisms have different p H optima. The experiments were carried out to optimize the p H. Degradation of phenol at various p H, 5 to 9, which plays a vital role in the growth, degradation and lysis of bacteria is shown in Fig.-2. The most favorable p H for the strain to achieve the maximum rate of phenol degradation was 7 whereas the phenol degradation at other p H values was slower.
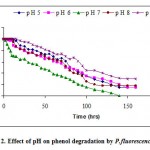 |
Figure 2: Effect of pH on phenol degradation by P.fluorescence.
|
Effect of temperature of the medium on phenol degradation
The high dependence of enzymatic activity and cellular maintenance requirements on temperature makes it an important quantity. Temperature exerts an important regulatory influence on the rate of metabolism. From the Fig. -3, the effect of temperature on phenol degradation by P fluorescence was assessed. The degradation rate increased with increase of temperature form 27 to 30°C. Above the optimal temperature, thermal death occurred so the specific growth and phenol removal rates decreased. The optimum temperature was 30°C at which the degradative enzyme reached the highest activity.
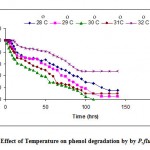 |
Figure 3: Effect of Temperature on phenol degradation by by P.fluorescence.
|
In order to determine the effect of phenol concentration on microbial growth and phenol removal rates a series of batch experiments were conducted in shake flasks by P. fluorescence at optimum conditions (inoculum size 5% v/v, p H 7, temperature 30°C). Experiments were conducted at various initial phenol concentrations ranging from 100-750 mg/l as shown in the Fig.-4 (All the data not shown for clarity). Results of these studies show that higher the concentrations of phenol the more time it takes to be degraded completely. At lower initial phenol concentrations P. fluorescence degrades phenol immediately with no lag phase indeed at phenol concentrations greater than 480mg/l there occurs a lag phase. At each of the phenol concentrations there was a period of exponential growth period which is further confirmed by substrate being consumed at faster rate.
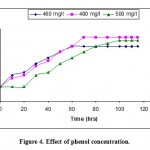 |
Figure 4: Effect of phenol concentration.
|
In summary, these results shows P. fluorescence can be tolerated up to 480 mg/l at these optimum values.
References
- Khan, A. and Walla, S. “Cloning a bacterial genes specifying degradation of 4-chlorobiphenyl from Pseudomonas putida 0483.” J. App. Env. Microbiol., 55: 798-805 (1989).
- Hutzinger, A. and Veerkamp, W. “Xenobiotic chemicals with pollution potential”. FEMS Sym. 12: 3-45 (1981).
- National Research Council, Committee on the Assessment of Polytechnical Biphenyls in the Environment: In poly chlorinated biphenyls, National Academy of Sciences, Washington, D.C. (1979).
- Environmental Protection Agency (EPA). Phenol ambient water quality criteria. Office of planning and standards. Washington, D.C. BB296786. (1979).
- Agency for Toxic Substances and Disease Registry (ATSDR). Medical Management Guidelines for Phenol. (2003).
- Annadurai, G., Rajehbabu, S., Mahesh, K.P.O. and Murugen, T. “Adsorption and Biodegradation of phenol by chitosan-immobilized Pseudomonas putida (NICM 2174).” Bioprocess Engineering, 22: 493-501 (2000).
- Hill, G.A. and Robinson, C.W. “Substrate inhibition kinetics: Phenol Degradation by Pseudomonas putida.” Biotechnol. Bioeng., 17: 599-1615 (1975).
- Li, J.K. and Humphrey, A.E. “Kinetics and fluorometric behavior of a phenol fermentation.” Biotechnol. Lett., 11: 177-182 (1989).
- Calabrese E.J. and Kenyon, E.M. Air toxics and Risk Assessment. Lewis Publishers, Chelsea, M.I. (1991).
- Prpich, G.P. and Daugulis, A.J. “Enhanced biodegradation of phenol by a microbial consortium in a solid-liquid two phase partitioning bioreactor.” Biodegradation, 16: 329-339 (2005).
- Goudar, C.T., Ganji, S.H., Pujar, B.G. and Strevett, K.A. “Substrate inhibition of phenol biodegradation.” Water Environ. Res., 72 : 50-55 (2000).
- Kobayashi, H. and Rittman, B.E. “Microbial removal of hazardous organic compounds.” Environ. Sci. Technol., 16 : 170-183 (1982).
- Usama Beshay, Desouky, Abd-EI-Haleem, Hassan Moawad and Sahar Zaki. “Phenol biodegradation by free and immobilized Acinetobacter.” Biotechnology Letters, 24: 1295-1297 (2002).
- Desouky Abd-EI-Haleem, Usama Beshay, Abdu O Abdelhamid, Hassan Moawad and Shar Zaki. “Effects of mixed nitrogen sources on biodegradation of phenol by immobilized Acinetobacter sp., strain W-17.” African Journal of Biotechnology, 2(1): 8-12 (2003).
- Arutchelvan,V., Kanakasabai, V., Nagarajan, S. and Muralikrishnan, V. “Kinetics of high strength phenol degradation using Bacillus brevis.” Journal of Hazardous Materials, B129 : 216-222 (2006).
- Balasankar, T. and Nagarajan, S. “Biodegradation of phenols by a plasmid free. Bacillus brevis.” Asian Journal of Microbiology, Biotechnology and Environmental Science, 2(3-4): 155-158 (2000).
- Salmeron-Alcocer, A., Ruiz-Ordaz, N., Juarez-Ramirez, C. and Galindez-Mayer, J. “Continuous biodegradation of single and mixed chlorophenols by a mixed microbial culture constituted by Burkholderia sp., Microbacterium phyllosphaerae, and Candida tropicalis.” Biochemical Engineering Journal,1-11 (2007).
- Arutchelvan,V., Kanakasabai, V., Nagarajan, S. and Muralikrishnan, V. “Isolation and identification of novel high strength phenol degrading bacterial strains from phenol- formaldehyde resin manufacturing industrial wastewater.” Journal of Hazardous Materials, 238-243 (2005).
- Tsuey-Ping Chung, Pei-Chen Wu, Ruey-Shin Juang. “Use of microporous hallow fibres for improved biodegradation of high-strength phenol solutions.” Journal of Membrane Science, 258: 55-63 (2005).
- Oboirien, B.O., Amigun, B., Ozumu, T.V., Ogunkunle, O.A., Adetunji, O.A., Betiku, E. and Solomon, B.O. “Substrate inhibition kinetics of phenol degradation by Pseudomonas aeruginosa and Pseudomonas fluorescence.” Biotechnology, 4(1): 56-61 (2005).
- Satish Kalme, Ganesh Parshetti, Sushma Gomare and Sanjay Govandwar. “Diesel and kerosene degradation by Pseudomonas desmolyticum NCIM 2112 and Nocardia hydrocarboxydans NCIM 2386.” Curr. Microbiol., 56: 581-586 (2008)
- Santos V.L. and Valter R. Linardi. “Biodegradation of phenol by a filamentous fungi isolated from industrial effluents-identification and degradation potential.” Process Biochemistry, 39(8): 1001-1006 (2004).
- Weijian Cai, Jiwu Li and Zhen Zhang. “The characteristics and mechanisms of phenol biodegradation by Fusarium species.” Online (2007).
- Salmeron-Alcocer, A., Ruiz-Ordaz, N., Juarez-Ramirez, C. and Galindez-Mayer, J. “Continuous biodegradation of single and mixed chlorophenols by a mixed microbial culture constituted by Burkholderia sp., Microbacterium phyllosphaerae, and Candida tropicalis.” Biochemical Engineering Journal, 1-11 (2007).
- Bastos, A.E.R., Tornisielo, V.L., Nozawa, S.R., Trevors, J.T. and Rossi, A. “Phenol metabolism by two microorganisms isolated from Amazonian forest soil amples.” Indian Journal of Microbiology, Biotechnology, 24(6): 403-409 (2000a).
- Yan Jiang, Jianping Wen, Xiaoqiang Jia, Qianggele Caiyan and Zongding Hu. “Mutation of Candida tropicalis by irradiation with a He-Ne laser to increase its ability to degrade phenol.” Applied Microbiology Biotechnology, 73 (1): 226-231 (2007).
- Gabriele Pinto, Antonino Pollio, Lucio Previtera and Fabio Temussi. “Biodegradation of phenols by microalgae.” Biotechnology letters, 24: 2047-2051 (2002).
- Schroder, M., Muller, C., Posten, C. Deckwer, W.D. and Hecht, V. “Inhibition kinetics of phenol degradation from unstable steady state data.” Biotechnol. Bioeng., 54: 567-576 (1997).
- Sivakumar, A. “Studies on microbial production of 2, 3- butanediol by K. oxytoca in batch continuous and cell recycle systems.” Ph.D. Thesis. IIT, Madras, India. (1995).
- American Public Health Association (APHA), American Water Works Association, Water Pollution ControlFederation. Standards Methods for the Examination of Water and Wastewater. 17th Edition. Washington, D.C. 9-55 – 9-62 (1989).

This work is licensed under a Creative Commons Attribution 4.0 International License.





