How to Cite | Publication History | PlumX Article Matrix
K. Ravi Shankar*, Sanjoy Das and Pavani Bujala
Sri Sai Aditya Institute of Pharmaceutical Sciences and Research, A.D.B. Road, Surampalem, Peddapuram - 533 437, East Godavari District India.
Corresponding Author E-mail: kravishankar_1963@yahoo.com.
ABSTRACT: The study was carried out to evaluate the qualitative analysis of phytochemicals and in vitro antibacterial activity of Dregea volubilis (L. f.) Benth. ex Hook. f. [Syn. Wattakaka volubilis (L. f.) Stapf., Marsdenia volubilis (L. f.) Cooke and Schollia volubilis (L. f.) Jacq. ex Steud.] (Family: Asclepiadaceae) leaf from its ethanol (continuous hot percolation by ethanol in soxlet apparatus) and aqueous (cold maceration in distilled water for 72 hours) extract. Dried ethanol extract was subjected to preliminary phytochemical screening for the presence of different phytoconstituents and was found to have saponins, coumarins and phytosterols. Ethanol and aqueous leaf extract (ELE and ALE respectively) were subjected to antibacterial screening by disc diffusion method against three gram positive and six gram negative bacteria. ELE was found to be active whereas ALE to be inactive.
KEYWORDS: ALE; antibiotic; antibacterial; Disc diffusion; Dregea volubilis; ELE; Marsdenia volubilis
Download this article as:| Copy the following to cite this article: Shankar K. R, Das S, Bujala P. Phytochemical Screening and In Vitro Antibacterial Activity of Ethanol and Aqueous Extracts of Dregea Volubilis Leaves. Biosci Biotech Res Asia 2010;7(2) |
| Copy the following to cite this URL: Shankar K. R, Das S, Bujala P. Phytochemical Screening and In Vitro Antibacterial Activity of Ethanol and Aqueous Extracts of Dregea Volubilis Leaves. Biosci Biotech Res Asia 2010;7(2). Available from:https://www.biotech-asia.org/?p=9641 |
Introduction
The spread of multi-drug resistant pathogens is one of the most serious threats to successful treatment of microbial diseases1. The increasing failure of chemotherapeutics and antibiotic resistance exhibited by pathogenic microbial infectious agents has led to screening of several medicinal plants for their potential antimicrobial activity2. Hence, newer herbal antibacterial compounds from plants and their semi-synthetic derivatives to overcome the resistance are under investigation3.
Alcoholic and hydro-alcoholic extracts of Dregea volubilis were reported to have anti-inflammatory, analgesic, anti-hyperlipidemic and hypoglycaemic activity in alloxan-induced diabetic rats4, 5, 6. A HPTLC method for quantification of Aeridin (2, 7-dihydroxy-1, 3-dimethoxy-9, 10-dihydrophenanthropyran), responsible for anti-inflammatory activity in the plant was developed and standardized7. Recently, an unusual novel triterpenoid ether, multiflor-7-ene-12, 13-ether and a new multiflor -7-ene-12α-ol were also isolated and characterized8. Three novel polyoxypregnane glycosides, volubiloside A, B and C (1–3), were isolated from the flowers of Dregea volubilis9.
The aim of the present study is to evaluate the anti-bacterial activity of Dregea volubilis leaf against various bacterial strains.
Material And Methods
Plant material
Dregea volubilis leaves were collected from Veravaram, East Godavari district, Andhra Pradesh, India. The plant was authenticated by scientist P. V. Prasanna in Botanical Survey of India, Deccan Regional Centre, Hyderabad and incorporated in the herbarium with voucher specimen number assigned as 000834. The leaves were washed with fresh water and dried under shade at room temperature. The leaves were powdered and stored. 60 gm of powdered drug was extracted separately with ethanol and water by continuous hot percolation in soxlet apparatus and cold maceration for 3 days respectively. Both the extracts were filtered and evaporated using a rotary evaporator. Dried extracts (ELE 12.98 %w/w and ALE 41.65 % w/w) were stored at 200C until used.
Phytochemical screening
Dried ethanol extract was subjected to screen for the presence of different phytoconstituents like amino acids, protein, lipid, anthraquinones, flavonoids, coumarins, saponins, cyanogenetic & cardioactive glycosides, tannins, catechin, phytosterols, alkaloids, gum and mucilage.
Test organisms
The test gram positive and negative organisms used were Staphylococcus aureus ATCC BAA 1026, Staphylococcus warneri ATCC 27836 & Bacillus subtilis ATCC 11774 and gram negative bacteria Escherichia coli ATCC 10536, Acinetobacter baumannii ATCC BAA 1794, Pseudomonas putida ATCC 700007, Pseudomonas aeruginosa ATCC 10662, Proteus mirabilis ATCC 14153 & Klebsiella pneumoniae ATCC 33495. All strains were collected from Microbes Speciality Culture Lab., above Dhanwantari blood bank, Near China Anjaneyaswamy Temple, Danavaipeta, Rajahmundry, East Godavari District- 533 103, Andhra Pradesh, India.
Determination of antibacterial activity
The test strains were suspended in nutrient broth (Human Diagnostic and Surgichem, Kolkata) and incubated for 24 hours at 37ºC10. Each extract was dissolved in its respective solvent to get a concentration of 1 gm/ml as stock solution. Stock solutions were sterilised using filtration sterilisation technique (Cellulose nitrate membrane filter, 0.45 µm, Whatman International Ltd., Maidstone, England) and kept separately for use. Sterile Whatman no. 1 filter paper discs of 6 mm diameter were loaded with 5 and 10 µl of each stock solution10, 11. All paper discs were allowed to evaporate before inoculation12. ELE and ALE loaded discs of both concentrations were inoculated separately to nutrient agar (Qualigens Fine Chemicals, Mumbai) of each plate previously swabbed with same bacterial cell suspension using sterile cotton swab. Positive control for every plate was performed by loading 10 µl of antibiotic solution (1 mg/ml) per disc [Ampicillin for E. Coli, A. baumanii, P. aeruginosa, P. putida & P. mirabilis Streptomycin for K. Pneumoniae & B. Subtilis and Amikacin for S. aureus & S. warneri]13, 14. Negative controls were served by loading 10 µl of corresponding solvent (ethanol and sterile distilled water) for every plate under each group of extract. Each organism for both ALE & ELE under a separate method without negative control disc was screened in a plate having discs of standard antibiotic (10 µg/disc) and three different concentrations of extract (5, 7.5 and 10 mg/disc) after drying. Plates were incubated overnight (18 hours) at 37ºC. At the end of the incubation period the antibacterial activity was evaluated by measuring the inhibition zones (diameter of inhibition zone plus diameter of disc)12.
Results
Ethanol extract was found to have saponins, coumarins and phytosterols. Inhibition zone is expressed separately for both the methods with and without negative control in Table 1 and Table 2 respectively. Some plates showing the inhibition zones are presented in Figure 1, 2 & 3 and Figure 4, 5 &6 for the methods with and without negative control respectively.
Table 1: Inhibition zone in method without negative control
| Bacteria | Inhibition zone (mm) | |||||||
| Positive control | ELE | ALE | ||||||
| ELE | ALE | T1 | T2 | T3 | T1 | T2 | T3 | |
| S. aureus | 18 | 19 | 13 | 14 | 15 | NI | NI | NI |
| S. warneri | 19 | 19 | 11 | 13 | 14 | NI | NI | NI |
| B. subtilis | 21 | 20 | 11 | 12 | 13 | NI | NI | NI |
| E. coli | 20 | 19 | 12 | 13 | 14 | NI | NI | NI |
| A. Baumannii | 19 | 18 | 13 | 14 | 14 | NI | NI | NI |
| P. aeruginosa | 20 | 21 | 14 | 15 | 17 | NI | NI | NI |
| P. putida | 20 | 21 | 16 | 17 | 18 | NI | NI | NI |
| K. pneumoniae | 20 | 20 | NI | NI | NI | NI | NI | NI |
| P. mirabilis | 21 | 22 | 12 | 13 | 14 | NI | NI | NI |
NI: no inhibition, T1: 5 mg/disc of ELE or ALE, T2: 10 mg/disc of ELE or ALE.
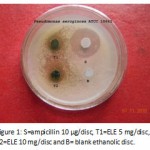 |
Figure 1: S=ampicillin 10 µg/disc, T1=ELE 5 mg/disc, T2=ELE 10 mg/disc and B= blank ethanolic disc.
|
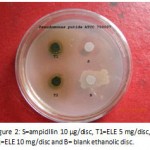 |
Figure 2: S=ampicillin 10 µg/disc, T1=ELE 5 mg/disc, T2=ELE 10 mg/disc and B= blank ethanolic disc.
|
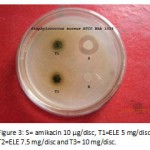 |
Figure 3: S= amikacin 10 µg/disc, T1=ELE 5 mg/disc, T2=ELE 7.5 mg/disc and T3= 10 mg/disc.
|
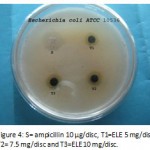 |
Figure 4: S= ampicillin 10 µg/disc, T1=ELE 5 mg/disc, T2= 7.5 mg/disc and T3=ELE 10 mg/disc.
Click here to View figure |
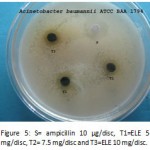 |
Figure 5: S= ampicillin 10 µg/disc, T1=ELE 5 mg/disc, T2= 7.5 mg/disc and T3=ELE 10 mg/disc.
|
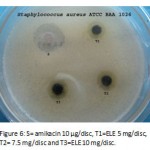 |
Figure 6: S= amikacin 10 µg/disc, T1=ELE 5 mg/disc, T2= 7.5 mg/disc and T3=ELE 10 mg/disc.
|
Discussion
Ethanol leaf extract showed significant antibacterial activity against both gram positive and negative strains whereas aqueous leaf extract was found to be inactive. In the method without negative control, the highest inhibition zone was 18 mm against P. putida followed by 17 mm against P. aeruginosa responsible for pneumonia, septic shock, urinary tract infection, gastrointestinal infection, skin & soft tissue infection especially in burns etc. and 15 mm against S. aureus responsible for staphylococcal scalded skin syndrome, furuncles, carbuncles, septic arthritis, atopic dermatitis, endocarditis, toxic shock syndrome, pneumonia, food poisoning & mastitis in cow. In the method with negative control, the highest inhibition zone was 17 mm against P. putida followed by 15 mm against P. auruginosa and 14 mm against A. baumannii responsible for pneumonia and severe life threatening infections such as necrotizing fasciitis.
Acknowledgement
The authors (K.R.S. and S.D.) would like to convey the greatest thanks to Sri N. Satish Reddy, VC of Aditya Educational Institutions for providing laboratory facilities and other needs. They are thankful to laboratory technicians V. Velankini, J. S. Maha Lakshmi and Y. L. Prasanna for their continuous help. They also express their sincere thanks to Botanical Survey India scientist P. V. Prasanna for authentication of the plant and Mr. Sanyasa Setti for supplying the crude drug from his native village.
References
- Chaudhry, N. M. A. and Tariq, P. “In vitro antibacterial activities of kalonji, cumin and poppy seeds.” J. Bot., 40: 461-7 (2008).
- Colombo, M. L. and Bosisio, E. “Pharmacological activities of Chelindonium majus L (Papaveraceae).” Res., 33: 127-34 (1996).
- Palaksha, M. N., Ahmed, M. and Das, S. “Antibacterial Activity of Garlic Extract Streptomycin-resistant Staphylococcus aureus and Escherichia coli solely and in synergism with Streptomycin.” J Nat. Sc. Biol. Med., 1: 12-5 (2010).
- Latha, P. G., Divya, T. S. and Usha, K. “Anti-inflammatory, analgesic and anti-lipid peroxidative properties of Wattakaka volubilis (Linn. f.) Stapf.” Prod. Rad., 8: 137-41 (2009).
- Arun Kumar, R., Ahmed, A. B. A., Venkateshvaran, Mani, P. and Jhon Bastin, T. M. M. “Anti-hyperlipidemic and hypoglycaemic activity of Wattakaka volubilis methanol extract in alloxan-induced diabetic rats.” Pharm. Res., 3: 1913-5 (2010).
- Nandi, D., Besra, S., E., Dey, S. and Giri, V. S. “Anti-inflammatory and analgesic activities of leaf extract of Wattakaka volubilis (Dregea volubilis).” J. Green Pharm.,3: 175-263 (2009).
- Mridula, G., Shreedhara, C. Katkar, K., Sutharand, A., Chauhan, S. A. and Singh, V. “A quantitative estimation of Aeridin in Wattakaka volubilis by HPTLC.” Pharm. Res., 3: 1913-5 (2010).
- Reddy, V. L. N., Ravikanth, V., Reddy. A. V., Rao, T. P. and Venkateshwarlu, Y. “A unusual novel triterpenoid ether, multiflor-7-ene-12, 13-ether and a new multiflor -7-ene-12α-ol from Wattakaka volubilis.” Tetrahedron Letters, 43: 1307-11 (2002).
- Sahu, N. P., Panda, N., Mandal, N. B., BAnerjee, S., Koike, K. and Nikaido, T. “Polyoxypregnane glycosides from the flowers of Dragea volubilis.” Phytochemistry, 61: 883-8 (2002).
- Benny, P. J. and Gopalakrishnan, S. “In vitro antimicrobial activities Punica granatum extract on bacteria causing urinary tract infections.” Indian Drugs, 46: 17-22 (2009).
- Abboud, M., Khleifat, K. M., Qaralleh, H. N. and Tarawneh, K. A. “Antibacterial activity in vitro of Thymus capitatus from Jordan.” J. Pharm. Sc., 22: 247-51 (2009).
- Tanti, B., Buragohain, A. K., Gurung, L., Kataki, D., Das, A. K. and Borah, S. P. “Assessment of antimicrobial and antioxidant activities of Dendrocnide sinuate (Blume) Chew leaves- A medicinal plant used by ethnic communities of North East India.” J. Nat. Pro. Resources, 1: 17-21 (2010).
- Government of India, Ministry of Health and Family Welfare, “Indian Pharmacopoeia,” Vol I, The Indian Pharmacopoeia Commission, Ghaziabad, 48 (2007).
- Karsha, P. V. and Lakshmi, O. B. “Antibacterial activity of Black pepper (Piper nigrum Linn.) with special reference to its mode of action on bacteria.” Indian J. Nat. Pro. Resources, 1: 213-5 (2010).

This work is licensed under a Creative Commons Attribution 4.0 International License.





