How to Cite | Publication History | PlumX Article Matrix
Eman M. A. El-Taher
The Regional Center for Mycology and Biotechnology, Al-Azhar University, Cairo, Egypt.
DOI : http://dx.doi.org/http://dx.doi.org/10.13005/bbra/1027
ABSTRACT:
Many useful properties of locally edible mushrooms are still being studied. This study shows for the first time, the green biosynthesis of silver nanoparticles (AgNPs), with the helping of the mycelial mat of local-edible mushroom; Pleurotus ostreatus. The present process is an excellent candidate for industrial scale production of nanoparticles. Hence, two modes of biosynthesis were identified: with the mycelia, extracellular and intracellular biosynthesis. The ability of P. ostreatus to produce AgNPs under sun light, instantaneously, is reported. Optical detection followed by confirmation through spectroscopic analysis suggests that this fungus can be used for the purpose of safe and sure synthesis of AgNPs. For morphological details of cell structure, transmission electron microscopic (TEM) analysis was carried out. TEM was also showed the nanoparticles, were mostly spherical, polydispersity and distribution of AgNPs in the range of 3 - 25 nm with an average size of 10 nm. EDX analysis recorded that, signal from the Ag atoms in the nanoparticles is observed, while, signals from C, O, Na, Si, P, S, Cl, K and Ca atoms were also recorded. The current research confirmed that, these nanoparticles had potent antimicrobial effect on pathogenic bacteria Bacillus subtilis and Escherichia coli and the pathogenic fungus Aspergillus niger. Moreover, focus has been given to the development of an efficient and eco-friendly viable process for sterilization of textile fabrics, which are using at ancient pharaonic works.
KEYWORDS: Mushrooms mycelia; Silver nanoparticles; Green synthesis; Antimicrobial activity
| Copy the following to cite this article: El-Taher E. M. A. Instantaneous Biosynthesis of Noble Metal (Ag) Nanoparticles Assisted by Edible Mushroom Biomass and Their Treatment of Textile Fabrics. Biosci Biotech Res Asia 2012;9(2) |
| Copy the following to cite this URL: El-Taher E. M. A. Instantaneous Biosynthesis of Noble Metal (Ag) Nanoparticles Assisted by Edible Mushroom Biomass and Their Treatment of Textile Fabrics. Biosci Biotech Res Asia 2012;9(2). Available from: https://www.biotech-asia.org/?p=9903 |
Introduction
The synthesis of metal nanoparticles is the current research trend because they exhibit different physical and chemical properties compared to their bulk metals (Gratzel, 2001 and Xia, et al., 2003). This is due to the large surface area obtained in the nanoparticles, where the chemical properties of the metals are intensified (Ray, et al., 2011). In the past decade there has been a tremendous amount of research interest in nano-materials with respect to its production, properties and applications (Narayanan & Sakthivel, 2010 and Singh, et al., 2011). Artificially made metal-NPs are typically produced on a small laboratory scale using methods such as chemical vapor deposition, irradiation or chemical reduction of metal salts. However, most of these processes give rise to harmful byproducts (Mansoori, 2005). Therefore, stress is laid on benign biosynthesis process which results in environment friendly nanoparticles of biological origin. The use of microorganisms as nano-factories enables us to use simple large scale production of nanomaterials, which does not give rise to toxic waste products. Microbial assisted biosynthesis of NPs is therefore a rapidly progressing area of nano-biotechnology (Jaidev & Narasimha, 2010).
Both unicellular and multicellular organisms are known to produce inorganic materials either intracellularly or extracellularly (Campbell, et al., 2001 and Smith & Hunt, 1985). The biosynthesis of inorganic nanomaterials using eukaryotic organisms such as fungi was achieved with the intracellular production of silver nanoparticles by Verticillium strains (Sastry, et al., 2003). Recently, it was found that aqueous silver ions may be reduced extracellularly using the fungus fF. oxysporum to generate AgNPs in water (Ahmad, et al., 2003). The nanoparticles could be stabilized directly in the process by proteins (Durán, et al., 2005). Up to date, extra- and intracellular synthesis of AgNPs by Pleurotus ostreatus, has not been reported and thus is reported here for the first time.
Ray, et al. (2011) concluded that production of nanoparticles using filamentous fungi has some advantages over other organisms. Filamentous fungi are easy to handle, require simple raw materials and has high wall-binding capacity. Different fungi, such as Verticillium, Fusarium oxysporum and Colletotrchum sp., have been reported by (Shankar, et al., 2003; Sastry, et al., 2003; Ahmad, et al., 2003; Fayaz, et al., 2009; Mukherjee, et al., 2001A and Mandal, et al., 2006) to synthesize metal nanoparticles.
Biosynthesis methods can be divided into three categories depending on the place where nanoparticles are created, i.e. intra, extracellular (Brewer, et al., 2007) and supernatant extract (Shahverdi, et al., 2007B). The use of fungi holds promise for large scale metal nanoparticles productions, as the enzymes secreted by fungi is an essential element for the biosynthesis of metal nanoparticles (Das & Marsili, 2010). Durán, et al. (2005) reported that, the reduction of the metal ions occurs by a nitrate-dependent reductase and a shuttle quinone extracellular process. In the biosynthesis of metal nanoparticles by a fungus, the fungus mycelium is exposed to the metal salt solution. That prompts the fungus to produce enzymes and metabolites for its own survival. In this process, the toxic metal ions are reduced to the none-toxic metallic solid nanoparticles, through the catalytic effect of the extracellular enzyme and metabolites of the fungus (Vahabi, et al., 2011).
The presence of uniformly distributed AgNPs on the surface of the fungal cells is also observed by Sastry, et al. (2003), indicating that the nanoparticles formed by the reduction of Ag+ ions, which are bound to the surface of the cells. The silver nanoparticles seen outside the mycelia may be due to weakly bound AgNPs dislodged from the biomass during preparation of the films for SEM investigation, which it may be recalled, involves washing the biomass thrice with distilled water. They were also included that, the location of the AgNPs relative to the fungal cells would be important in elucidating the mechanism of their formation. A number of dark spots on the cell walls, as well as some spots within the cytoplasm were shown. These dark spots correspond to AgNPs synthesized by the fungus predominantly on the cell wall. Moreover, silver particles clearly bound to the surface of the cytoplasmic membrane.
Durán, et al., 2007 reported that, time-dependent increase in the intensity of the plasmon resonance, at wavelength 440 nm, confirming the AgNPs formation. The appearance of a brownish color in solution containing the biomass is a clear indication of the formation of AgNPs in the reaction mixture.
The initial approach of mycosynthesis of AgNPs was carried out by challenging an acidophilic pathogenic fungus with silver nitrate (AgNO3) leading to the reduction and accumulation of AgNPs of about 25 nm in diameter intracellularly within the biomass (Mukherjee, et al., 2001B). Beside this other silver tolerant fungi can produce AgNPs of different sizes but with spherical shapes. However, Aspergillus fumigatus can produce both spherical and triangular shaped silver nanoparticles of size 5-25 nm (Bhainsa & D’Souza, 2006). Later on a number of fungi have been investigated by Acharya, et al. (2009) and were found to be capable of biosynthesizing AgNPs having different particle size and shape, both extra and intracellularly.
EDAX (energy dispersive analysis of x-rays) spectrum recorded by Sastry, et al. (2003) in the spot-profile mode from one of the densely populated AgNPs regions on the surface of the fungal cells. Strong signals from the silver atoms in the nanoparticles are observed, while weaker signals from C, O, S, P, Mg and Na atoms were also recorded. They concluded that, the C, O, S, P, Mg and Na signals are likely to be due to X-ray emission from proteins/enzymes present in the cell wall of the biomass.
In previous studies by Slawson, et al. (1992); Spadaro, et al. (1974); Stoimenov, et al. (2002) and Zhao & Stevens (1998), silver has demonstrated antimicrobial activity against a broad range of fungi, viruses, and bacteria. As silver salts, having an antimicrobial effect (Silver & Phung, 1996), synthesis of silver nanoparticles now a days are of great desire (Saha, et al., 2010). The mechanism of antimicrobial property of nanoparticle lies with the fact that the extremely small size means a large surface area relative to the volume, which effectively covers the microorganisms and reduce oxygen supply for respiration. Silver nanoparticles are, therefore, a safer alternative to antibiotics (Jain, et al., 2009). Sondi & Salopek-Sondi (2004) evaluated the antimicrobial activity of silver nanoparticles against Escherichia coli. The results confirmed that the treated E. coli cells were damaged, showing pit formation on bacterial cell walls. Falkiewicz-Dulik & Macura (2008) studied the antifungal activity of AgNPs for the usefulness of nanosilver in bio-stabilization of footwear materials. The 1% solution inhibited the growth of the majority of yeast-like fungal and mold strains. Silver nanoparticles at 100 ppm totally inhibited bacterial growth, but the activity against molds and dermatophytes was lower, and it was reported that molds and bacteria were resistant to 50 ppm of silver nanoparticles. Kim, et al. (2008 & 2009) reported that, spherical AgNPs showed potent activity against Trichophyton mentagrophytes, Trichosporon beigelii and Candida albicans compared with that of commercially available antifungal agents (amphotericin B and fluconazole). Stable colloidal solutions containing up to 35 ppm nanoparticles were found to have effective antifungal properties against Aspergillus, Penicillium and Trichoderma species (Petica, et al., 2008).
In the last few decades, there has been increased interest in reducing the availability of commercial textile containing antibacterial agents due to environmental pollution. Since silver is a good antibacterial agent and non-toxic and natural inorganic metal, it appears as an interesting material to be used in different kind of textile fibers. In this direction, polypropylene/silver nano-composite fibers were prepared and the antibacterial tests showed that the fibers containing silver nanoparticles in core-part (inside the fiber) had no nearly significant antibacterial activity. However, the fibers having silver nanoparticles (30 nm size) in sheath-part showed excellent antibacterial effects (Y eo, et al., 2003 and Y eo & Jeong, 2003). Textile fabrics with antibacterial efficacy were easily achieved using nanosized colloidal silver particles (2–5 nm size), by padding process on cotton and polyesters. These fabrics showed laundering durability against S. aureus and K. pneumoniae (Lee, et al., 2003). Similar results were achieved by Lee & Jeong (2004) with nanosized colloidal AgNPs on polyester nonwovens. The growth of bacteria colonies was absolutely inhibited with only 10 ppm colloidal silver when the mean diameter of the silver particles was 2–5 nm. Consequently, a smaller particle size yielded better bacteriostasis on silver-padded nonwoven fabrics. Silver nanoparticles can be coated onto polyurethane foams in diverse forms. This material can be washed several times without any loss of nanoparticles.
Experimental details
Materials
Culture of P. ostreatus (Oyster mushroom) used for synthesis of AgNPs is obtained friendly from the Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar University, Cairo, Egypt. These cultures were grown on potato dextrose agar (PDA) medium at 25°C for 6 days and maintained at 4°C in a refrigerator. Silver nitrate (AgNO3) was used in this study without further purification and the aqueous solution was made using distilled deionized water (DDW).
Silver nanoparticles preparation
Preparation of P. ostreatus mycelial mats
The mycelium of P. ostreatus was cultured, in vitro, on potato dextrose agar (PDA) and was used for production of AgNPs. For liquid culture, freshly cultivated mycelium from solid substrate was inoculated in 50 ml potato dextrose broth in 250 ml flasks and agitated at 28°C in dark. The biomass was harvested after 72 hours of growth. The biomass was washed with sterilized distilled water to remove medium component Ray, et al. (2011). Then, the biosynthesis of silver nanoparticles using the biomass was carried out.
Biosynthesis of silver nanoparticles
In the silver reduction, the two methods were followed Durán, et al. (2005).
Method A
For the synthesis of silver nanoparticles, approximately 10 g of P. ostreatus biomass was re-suspended in 100 mL distilled water in a conical flask and agitated for 72 h at 28°C. The resulting cell filtrate was collected by passing it through Whatman filter paper no.1 and this filtrate was used for nanoparticles synthesis. The cell free filtrate was challenged with AgNO3 (1mM) and the system was kept for several hours under sun light. A positive control (filtrate without the silver ion) and a negative control (aqueous solution of silver nitrate) were also run along with the experiment. The reduction of silver ions was confirmed by measurement of absorbance using UV–visible spectrophotometer.
Method B
To illustrate the effect of NPs production on the fungal cell ultrastructure using TEM, another test was also carried out as following: 10 g of P. ostreatus biomass was taken in 250 mL Erlenmeyer conical flask containing 100 mL of DDW. AgNO3 (1mM) was added to the flask and the reaction was carried out. Then, the ultrathin sections were cut using an ultra-microtome (Leica) and the sections were slightly stained with uranyl acetate and lead citrate prior to TEM analysis. TEM measurements were carried out on a JEOL JEM 1010 instrument, at RCMB, operated at an accelerating voltage of 70 kV. This low operating voltage was used to minimize damage to the thin sections by electron beam heating.
Characterization of silver nanoparticles
UV-visible spectroscopic analysis of AgNPs
The reduction of silver ions (Ag+) in aqueous solution of 1mM AgNO3 to silver nanoparticles (Ag0) was verified by qualitative testing of supernatant using UV–visible spectrophotometer at 420 nm. Periodically, 1ml of sample supernatant was withdrawn after 4, 24, 48, 72 … hrs and the absorptions were recorded.
Transmission electron microscopic analysis of AgNPs
For TEM analysis of particle shape, size and its distribution, a drop of aqueous suspension (50 μl) containing biosynthesized AgNPs was placed on the carbon-coated copper grids and allowing the water to evaporate before loading onto a specimen holder. TEM micrographs were taken by analyzing the prepared grids on JEOL TEM instrument. The TEM measurement of particle sizes was done by Image Analyzer System (IAS).
Scanning electron microscopic analysis of AgNPs
Energy-dispersive x-ray (EDX) microanalysis examination was performed to confirm the presence of silver in the particles, as well as to detect the other elementary compositions of the particles. EDX analysis at 20 KV was carried out by x-ray micro-analyzer (Module Oxford 6587 INCA X-sight) attached to JEOL JSM 5500 LV scanning electron microscopy, at RCMB.
Antimicrobial assays of Silver Nanoparticles
AgNPs suspended in deionized water were examined for their antimicrobial activity by the agar well diffusion technique (Damyanova, et al., 2000). The antibacterial activity of sample was studied against the pathogenic Gram +ve bacteria; Staphylococcus aureus (RCMB 010028), Bacillus subtilis (RCMB 010067) and Gram -ve bacteria; Pseudomonas aeruginosa (RCMB 010043) and Escherichia coli (RCMB 010052). Fresh overnight cultural inoculum of each organism was spread onto nutrient agar plates. The tested sample (100 µl) was loaded into the wells (6 mm diameter) of the plates. Mean zone of inhibition in mm ± standard deviation was measured. Ampicillin and Gentamicin were used as G+ve and G-ve antibacterial standard drugs, respectively. Antifungal activity of sample was screened against various pathogenic fungi; Aspergillus niger (RCMB 02596), Penicillium italicum (RCMB 03924), Candida albicans (RCMB 05031) and Geotricum candidum (RCMB 05097), using Sabourad dextrose (SDA) medium. The fungal cultures (0.1 ml) were spread out uniformly on the SDA plates, then, the tested sample was loaded into the wells of the plates. Each inhibition zone was measured three times to get an average value. Amphotericin B was used as antifungal standard drug.
Determination of minimal inhibitory concentration (MIC): Sterile DDW was used as the control and to dilute the culture of each organism to 105-106 CFU/ml. The required volume of test sample AgNPs (produced as above) at different concentrations (two-fold serial dilutions) were added and mixed well. The MIC was considered to be the lowest concentration that completely inhibits the inoculums comparing with the control.
Loading of Silver Nanoparticles on Fabrics
The antifungal properties of three AgNPs-coated natural fiber structures (Linen, Tents, Lenoh) using in the ancient pharaonic works, were examined. Fabrics were washed, sterilized and dried before use. Experiments were performed on samples with dimensions 5 × 5 cm. The final filtrate (100 ml) obtained above was treated by ultracentrifugation for 5 minutes and the filtrated superior part was eliminated to concentrate the AgNPs. In order to impregnate fabrics, these were submersed in an Erlenmeyer (50 ml) and shaking at 600 rpm for 24 h and dried at 70 oC. Inoculation test with Aspergillus niger was used to assess the ability of these textiles to inhibit fungal growth. Control fabrics without AgNPs were also included. After incubation for 48 h at 28 oC, the fabrics were analyzed by SEM after previously coated with gold, using SPI-Module sputter coater. The samples examined by SEM (JEOL JSM-5500 LV) JEOL Ltd, Japan, under low vacuum mode, at RCMB (Durán, et al., 2007).
Results and discussion
Preparation of Silver Nanoparticles
After addition of silver nitrate (1mM), the mycelia free filtrate (Method A) showed a gradual change in color under sun light with time from yellowish (Fig. 1 a) to reddish brown and finally to dark brown within 24 hours (Fig. 1b). The appearance of a reddish-brown color in solution containing the fungal filtrate is a clear indication of the formation of silver nanoparticles in the reaction mixture. These findings corroborate the results obtained by (Bhat et al., 2011) who reported that, metallic silver nanoparticles formed within a few minutes under sun light when (AgNO3) salt was added to the cell free filtrate. The protein present is further believed to cap the AgNPs formed, and thus they become functionalized and stable.
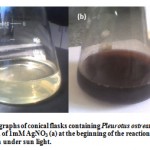 |
Figure 1: Digital photographs of conical flasks containing Pleurotus ostreatus cell free filtrate in aqueous solution of 1mM AgNO3 (a) at the beginning of the reaction and (b) after several hours of reaction under sun light.
|
From published data by Sanghi & Verma (2009), it is known that nanoparticles exhibit brown color in aqueous solution due to excitation of surface plasmon resonance (SPR) or vibrations, essentially the vibration of the group conduction electrons in the silver nanoparticles (Vahabi, et al., 2011 and Shankar, et al., 2004). Bhainsa & D’Souza (2006) had investigated extracellular biosynthesis of AgNPs was quite fast and they were formed within minutes of silver ion coming in contact with the cell filtrate.
Sanghi & Verma (2009) reported that, the fungus Coriolus versicolor when challenged with silver nitrate solution, silver NPs accumulated on its surface in 72 h which could be reduced to 1 h by tailoring the reaction conditions. Under alkaline conditions, the reaction was much faster and could easily proceed at room temperature even without stirring. Their study disclosed that, the amino groups were bound to the particles, which was accountable for the stability of NPs. It further confirmed the presence of protein as the stabilizing and capping agent surrounding the AgNPs. Experiments were conducted both with, media in which fungus was initially harvested and that of pristine fungal mycelium alone. Under normal conditions, in the case of media extracellular synthesis took place whereby other than the fungal proteins, glucose was also responsible for the reduction. In the case of fungal mycelium, the intracellular formation of AgNPs, could be tailored to give both intracellular and extracellular AgNPs under alkaline conditions whereby the surface S–H groups of the fungus played a major role.
TEM ultrastructure of fungal cells
Exposure of the P. ostreatus biomass to aqueous Ag+ ions (Method B) resulted in an intracellular reduction of the metal ions and formation of silver nanoparticles. Similarly, the Erlenmeyer flasks with its mat were a pale yellow color before the addition of Ag+ ions and this changed to a brownish color on completion of the reaction with Ag+ ions (data not shown). The appearance of a yellowish brown color in solution containing the fungal biomass suggested the formation of AgNPs (Sastry, et al., 1998 and Vahabi, et al., 2011).
Information on the location of the AgNPs relative to the fungal cells would be important in elucidating the mechanism of their formation and may be obtained by TEM analysis of thin sections of the P. ostreatus cells. Figure 2 shows, representative TEM images at various magnifications of the fungal cells; without Ag+ ions as a control (a, b & c) and with Ag+ ions (d, e & f). At lower magnification 5k (a & d), a number of hyphae can be observed, which on closer examination reveal assemblies of P. ostreatus cells within the hyphae. Morphological changes of the cells and accumulation of dark particles were observed in the image d. Moreover, this change was also observed in cells containing Ag atoms, as shown in (e & f) at high magnifications (30 & 80 k, respectively), demonstrating the influence of the silver in the morphology of these cells. Wherein, these images show a number of dark spots on the cell walls and clearly bound to the surface of the cytoplasmic membrane, as well as some spots within the cytoplasm, compared with the control sample (b & c) at the same magnifications. Similar result was observed by Sastry, et al. (2003) and concluded it is possible that, some Ag+ ions diffuse through the cell wall and are reduced by enzymes present on the cytoplasmic membrane and within the cytoplasm. It may also be possible that some of the smaller AgNPs diffuse across the cell wall to be trapped within the cytoplasm. Apparently, the AgNPs are concentrated at the surface and probably in the cytoplasm of the fungal cells. Mukherjee, et al. (2001B) recorded that, the dark spots correspond to AgNPs synthesized by the fungus predominantly on the cell wall. A number of silver particles can be seen on the mycelia wall surface, below the cell wall surface and affected the cell structures. However, the metal ions were not toxic to the fungal cells where the cells continued to multiply after biosynthesis of the AgNPs. They concluded that, the AgNPs were formed below the cell wall surface possibly due to reduction of the metal ions by enzymes present in the cell wall membrane. The exact mechanism leading to the intracellular formation of gold (Mukherjee, et al., 2001A) and silver (Mukherjee, et al., 2001B) nanoparticles by challenging the fungus with the corresponding metal ions, is not fully understood at the moment.
Sastry, et al. (2003) speculate that, since the nanoparticles are formed on the surface of the mycelia and not in solution, the first step involves trapping of the Ag+ ions on the surface of the fungal cells possibly via electrostatic interaction between the Ag+ and negatively charged carboxylate groups in enzymes present in the cell wall of the mycelia. Thereafter, the silver ions are reduced by enzymes present in the cell wall leading to the formation of silver nuclei, which subsequently grow by further reduction of Ag+ ions and accumulation on these nuclei.
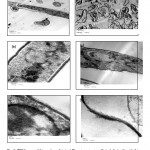 |
Figure 2: TEM images of thin sections of stained Pleurotus ostreatus cells (a, b & c) without Ag+ ions (as controls) and (d, e & f) after reaction with Ag+ ions for 24 h, at different magnifications (5k for a & d, 30k for b & e and 80k for c & f). Scale bars in (a & d), (b &e) and (c & f) corresponded to 2 µm, 500 nm and 100 nm, respectively.
|
Characterization of Silver Nanoparticles
Ultraviolet-Visible (UV-Vis) Spectroscopic analysis: The fungal cell filtrate after addition of aqueous AgNO3 (1 mM), to form AgNPs, was subjected to optical measurements by UV-Vis spectrophotometer. This analysis showed an absorbance peak at 420 nm, which was specific for the AgNPs (Gajbhiye et al., 2009 and Saha, et al., 2010). The absorbance recorded from the P. ostreatus reaction vessel, at different time intervals (4, 24, 48, 72 … hrs) of reaction, are reported in Table (1) and plotted in Figure (3). The strong surface plasmon resonance centered at 420 nm, is characteristic of colloidal silver, essentially the vibration of the group conduction electrons (Vahabi, et al., 2011). It might arise from the excitation of a longitudinal plasmon vibration of AgNPs in the solution (Henglein, 1993). However, Ahmad, et al. (2003) and RAY, et al. (2011) reported that time-dependent increase in the intensity of the plasmon resonance, which was observed in the fungal reaction vessels and confirming the AgNPs formation, was recorded at 440 nm. The absorbance was clearly showed the increase in intensity of silver solution with time, indicating the formation of increased number of AgNPs in the solution. According to this figure, there is no appreciable change in the UV-Vis absorbance of the reaction product after 168 hours, indicative of the fact that reaction came to equilibrium at about 2 days. It should be pointed out that the reaction was allowed to proceed for about 1-2 month (s). Interestingly, the solution was extremely stable even after a month of reaction (Data not shown) and this is par with Vahabi, et al. (2011). The exact mechanism of the synthesis of AgNPs was not known, but later it was hypothesized that the silver ions required the NADH-dependent nitrate reductase enzyme for their reduction, (Labrenz, et al., 2000 and Roh, et al., 2001) which was secreted by the fungus in its extracellular environment. The presence of NADH-dependent nitrate reductase enzyme in extracellular cell filtrate of the fungus used for the synthesis of nanoparticles has been confirmed, and the mechanism has been studied by Ingle, et al. (2008) and Anilkumar, et al. (2007).
Table 1: Relative absorbance at 420 nm of Pleurotus ostreatus
| Time (hrs) | 0 | 4 | 24 | 48 | 72 | 96 | 120 | 144 |
| Absorbance | 0 | 0.16 | 0.38 | 0.40 | 0.42 | 0.36 | 0.43 | 0.42 |
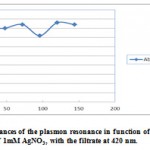 |
Figure 3: Intensity absorbances of the plasmon resonance in function of time of reaction in an aqueous solution of 1mM AgNO3, with the filtrate at 420 nm.
|
Morphological studies of silver nanoparticles: Exposure of the fungal filtrate to aqueous Ag+ ions resulted in the bioreduction of the metal ions and formation of AgNPs. The diameters of them were measured, using the Image Analyzer System, and the sizes obtained were in the range from 3 to 25 nm, while, the average being 10 nm. While, Ray, et al. (2011) reported that scanning and transmission electron microscopy showed particles were mostly spherical with a few of hexagonal shape. With TEM software, histogram was constructed showing the size range of the particles to be of 5 – 50 nm in diameter and the average being 21.91nm. However, Sastry, et al. (2003) reported that, analysis of the size of the AgNPs from many different TEM images of the Ag nano-Verticillium cells yielded a particle diameter of 25 ± 12 nm. The representative TEM picture recorded from the AgNPs film deposited on a carbon coated copper TEM grid is shown in Figure 4. This picture depicts individual AgNPs, as well as a number of aggregates. The morphology of the nanoparticles is highly variable, but the shape appears to be mostly spherical. Under observation of such images, these assemblies were found to be aggregates of AgNPs. Vahabi, et al. (2011) reported that, the nanoparticles were not in direct contact even within the aggregates, indicating stabilization of the nanoparticles by a capping agent. The separation between the AgNPs seen in the TEM image could be due to capping by proteins and would explain the UV-Vis spectroscopy measurements, which is characteristic of well-dispersed AgNPs. They are crystalline, as can be seen from the selected area diffraction pattern recorded from one of the nanoparticles in the aggregates.
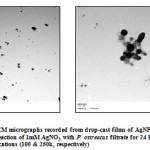 |
Figure 4(a & b): TEM micrographs recorded from drop-cast films of AgNPs solution formed by the reaction of 1mM AgNO3 with P. ostreatus filtrate for 24 hours, at different magnifications (100 & 250k, respectively) |
EDX analysis: The presence of silver is further confirmed by EDX by the presence of optical absorption peak in the range of 3 to 4 keV. An elemental composition analysis showed the presence of a signal from silver atoms, its mean percentage is equal 4.04 (Figure 5 & table 2). Magudapathy, et al. (2001) recorded that, the presence of optical absorption peak in the range of 3 to 4 keV, is typical for the absorption of metallic silver nano-crystallites. This analysis indicated that, the nano-structures were composed solely of silver with the noticeable contaminant being C, O, Na, Si, P, S, Cl, K, and Ca, presumably from the medium. They suggesting that, they were mixed precipitates from the centrifuged mycelia free media (MFM).
 |
Figure 5: with Table 2: A representative EDX spectrum of the synthesized AgNPs. The peak between 3 and 4 keV, is confirming the presence of silver. Different x-ray emission peaks are labeled.
|
Antimicrobial property
Investigation of the growth inhibition effect of AgNPs solution, against different microbes, was carried out and the results are depicted in table (3).
Antibacterial tests were performed against test organisms on petri plates by diffusion method. Stock solution of AgNPs had a range of specificity towards Bacillus subtilis (RCMB 010067), followed by Escherichia coli (RCMB 010052), with inhibition zone diameters equal 19.4 mm and 12.0 mm, respectively. While, the standard antibiotics, ampicillin and gentamicin showed mean inhibition zones 32.4 mm and 22.3 mm, respectively. The metal capture by bacteria was already described in the literature of Durán, et al. (2007). They reported that, it can be related with the presence of the cyanide or with atomic oxygen adsorption by the particles. Campbell et al. (2001) verified that, cyanide is formed as a secondary metabolite and that for the leaching process to occur, cyanide must be in close association with the silver surface leading to the silver oxidation and its posterior solubilization. Silver nanoparticles had potent antimicrobial effect on human bacteria E.coli (DH5α). The nanoparticles had potent inhibitory effect on the multi-drug resistant (MDR) pathogenic bacteria. Thus this source of AgNPs has the potential to be produced on a large scale and find application in the field of medicine and crop protection (Ray, et al., 2011). In the in vitro antifungal activity, amphotericin B (an antifungal agent that is widely used against many fungal infections) was used as positive control, for comparison with AgNPs. In the agar diffusion assay, antifungal standard showed mean inhibition zone 23.7 mm, while, it was 15.3 mm for AgNPs against A. niger (RCMB 02596). Kim et al. (2009) reported that, nano-Ag showed a significant effect against C. albicans when amphotericin B was used as a positive control. They also found that, it was also active against dermatophytic fungi like Trichophyton mentagrophytes. Bhat et al. (2011) concluded that, the bio-functionalized AgNP shows excellent antimicrobial property, either by mixing with antibiotic drug or by using directly as a drug. A survey of recent literature (Gajbhiye et al., 2009) showed remarkable findings on the bactericidal activity of (AgNPs) (Shahverdi, et al., 2007A and Kim, et al., 2007). Ingle, et al. (2008) found that AgNPs exhibited significant antimicrobial activity against E. coli and multi-drug-resistant Staph. aureus. Pal, et al. (2007) reported that the antibacterial activity of AgNPs against the gram-negative E. coli depends on the shape of the nanoparticles. Antimicrobial property of AgNPs, is due to close attachment of the nanoparticles surface with fungal cells and hence, its antimicrobial property is size dependent.
Minimal inhibitory concentrations. AgNPs has been tested for the MIC property using cultures of the same pathogenic bacteria and fungi, which were susceptible to the stock AgNPs solution (Table 3). Different concentrations of this solution (two-fold serial dilutions) were prepared. From the result, it is noted that AgNPs synthesized from mushroom was showing decent bactericidal activities against Bacillus subtilis (RCMB 010067) and Escherichia coli (RCMB 010052). Only 0.007 and 1.95 μg/ml standard antibacterials (ampicillin and gentamicin), respectively, were sufficient for the complete prevention of bacterial growth of Bacillus subtilis (RCMB 010067) and Escherichia coli (RCMB 010052), whereas in case of AgNPs, 15.6 and 125.0 μg/ml, respectively, were to be added for the growth inhibition. It is observed that the MIC of AgNPs was poor and more quantity is required for complete growth reduction of bacterial colony. On evaluation of the antimicrobial property of the produced AgNPs by Saha, et al. (2011) against Bacillus subtilis, Bacillus cereus, Escherichia coli, Proteus vulgaris, Pseudomonas aeruginosa and Micrococcus luteus, by cup plate method, showed specificity at a concentration of 100 μg/ml against the pathogenic bacteria. Bhat et al. (2011) measured the MIC to investigate growth inhibition effect of AgNPs solution against different microbes, namely, Staphylococcus aureus, Salmonella typhi, Providencia alcalifaciens and Proteus mirabilis. From their results, it is noted that AgNP synthesized from mushroom is showing decent bactericidal activity against gram +ve bacteria, especially in case of P. mirabilis, 2.5 μg/ml is sufficient for complete effect for reduction of bacterial colony and in case of gram −ve bacteria the antimicrobial activity of AgNP is moderate. In case of A. niger (RCMB 02596), the fungicidal activity of AgNPs was 62.5 µg / ml, compared with 1.98 µg / ml of the standard amphotericin B. (Panáčeka, et al., 2009) reported that, the antifungal activity of the AgNPs was evaluated for pathogenic Candida spp. by means of the determination of the MIC, minimum fungicidal concentration (MFC) and the time-dependency of yeast growth inhibition. The AgNPs exhibited inhibitory effect against the tested yeasts at the concentration as low as 0.21 mg/L of Ag. The inhibitory effect of silver NPs was enhanced through their stabilization and the lowest MIC equal to 0.05 mg/L was determined for AgNPs stabilized by sodium dodecyl sulfate against Candida albicans.
Table 3: Antimicrobial activity and MIC of the AgNPs against the tested organisms. The values were expressed as the diameter of inhibition zones in mm and concentrations in µg/ml.
|
Tested samples
Tested organisms
|
AgNPs (mm) |
Standard |
MIC (µg/ml) |
Standard |
| Fungi | Amphotericin B | Amphotericin B | ||
| A. niger (RCMB 02596) | 15.3±0.25 | 23.7±0.10 | 62.5 | 1.98 |
| P. italicum (RCMB 03924) | 0 | 21.9±0.12 | 0 | 3.9 |
| C. albicans (RCMB 05031) | 0 | 19.8±0.20 | 0 | 7.81 |
| G. candidum (RCMB 05097) | 0 | 28.7±0.22 | 0 | 0.06 |
| Gram +ve bacteria | Ampicillin | Ampicillin | ||
| S. aureus (RCMB 010028) | 0 | 27.4±0.18 | 0 | 0.12 |
| B. subtilis (RCMB 010067) | 19.4±0.64 | 32.4±0.10 | 15.6 | 0.007 |
| Gram -ve bacteria | Gentamicin | Gentamicin | ||
| P. aeruginosa (RCMB 010043) | 0 | 17.3±0.15 | 0 | 31.25 |
| E. coli (RCMB 010052) | 12.0±0.64 | 22.3±0.18 | 125.0 | 1.95 |
(0) = Resistant.
(±) = standard error between the three replicates.
Incorporation of AgNPs in Fabrics and Their Antifungal Effect
The growth of microbes on textiles during use and storage negatively affects the user as well as the textile itself. Consumers’ attitude towards hygiene and active lifestyle has created a rapidly increasing market for antimicrobial textiles, which in turn has stimulated intensive research and development. The detrimental effects can be controlled by incorporating the AgNPs colloid into fibers. In order to incorporate silver nanoparticles in the fabrics, these were immersed in the fungal filtrate, centrifuged and dried. The fungiostatic activity of the silver nanoparticles, impregnated the three types of fabrics (Linen, Tents, Lenoh), against Aspergillus niger was studied and the activity was indicated by a reduction of fungal growth. The fabrics incorporated with and without AgNPs were evaluated and analyzed by SEM. In the Linen fabric without AgNPs (control), a significant fungal growth as shown in Figure 6 (A) was observed. However, the same fabric with AgNPs, presented antifungal activity and showing no fungal growth in this one (Fig.6 (B)). In this figure of the fiber containing AgNPs, the absence of the contamination with fungus was observed. In the other hand, in the Tents and Lenoh fabrics, activity was not observed demonstrating a low incorporation of the AgNPs. This is may be due to the incorporation of AgNPs in the tissues is size dependent (Data not shown). This result demonstrated that AgNPs can be used to turn sterile specific types of fabrics. Marcato, et al. (2005) reported that Antibacterial activity was observed when silver nanoparticles were incorporated in cotton cloth. However, in silk cloth, this activity was not observed demonstrating a low incorporation of the AgNPs due possibly to the size of the silk pores. This work demonstrates the possible use of biological synthesized AgNPs and its incorporation in cloths leading them to sterilization. Moreover, these particles could have innumerable applications, in different areas as receptors, catalysis, biolabelling and others.
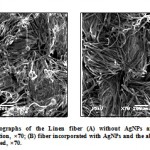 |
Figure 6: SEM micrographs of the Linen fiber (A) without AgNPs and showing fungal contamination, ×70; (B) fiber incorporated with AgNPs and the absence of the fungus was observed, ×70.
|
Conclusions and future directions
In summary, a brief overview of the use of fungi in the biosynthesis of AgNPs has been described. This is a new and rational biosynthesis strategy that is being developed. Extracellular secretion of enzymes offers the advantage of obtaining large quantities in a relatively pure state and can be easily processed by filtering of the cells. Compared to bacterial fermentations, in which the process technology involves the use of sophisticated equipment for getting clear filtrates from the colloidal broths, fungal broths can be easily filtered by filter press of similar simple equipment, thus saving considerable investment costs for equipment. The production of AgNPs occurred within the fungal biomass as well as extracellularly is an interesting feature of this particular fungus P. ostreatus. The high purity and the low toxicity of mushroom filtrate, render the synthesized AgNPs, potentially attractive for biological applications. Further, it can be concluded that, AgNPs can be used as effective agents against microbial pathogens. However, exhaustive experimental trials on animals are needed before using AgNPs as potential antimicrobial agents. Although there are lots of publications on biological synthesis of AgNPs using bacteria or fungi, the process is rather slow for complete reduction to produce AgNPs. The tested fungus, Pleurotus ostreatus was able to produce instantaneously, under sun light, thus indicating that the process is rather fast for complete bio-reduction to produce AgNPs. These AgNPs were of high purity, making them potentially useful for biological applications. This article reviews the application method of anti-contaminant agent AgNPs of Linen textile (using in the ancient pharaonic works). Thus these nanoparticles can be used both by the medicine industry as well as for control of fungal contamination of fabrics.
References
- Ahmad A., Mukherjee P., Senapati S., Mandal D., Khan M.I., Kumar R., Sastry M. (2003). “Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum,” Colloids and Surfaces B: Biointerfaces, 28: 313-318
- Acharya K., Sarkar J., Deo S. S. (2009). In: Bhowmik P.K., Basu S.K., Goyal A. (ed.) Bentham E-Books. Advances in Biotechnology, Bentham Science Publishers Ltd., p. 204-215
- Anilkumar S., Abyaneh M.K., Gosavi S.W., Kulkarni S.K., Pasricha R., Ahmad A. (2007). Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol Lett; 29:439-45
- Bhainsa K.C., D’Souza S. F. (2006). Colld. Surf. B: Biointerfaces 47 160–164
- Bhat R., Deshpande R., Sharanabasava V. Ganachari, Do Sung Huh, Venkataraman A. (2011). Photo-irradiated biosynthesis of AgNPs using edible mushroom Pleurotus florida and their antibacterial activity studies. Bioinorganic Chemistry and Applications, Volume 2011, 7 pages
- Brewer M., Zhang T., Dong W., Rutherford M., Tian Z.R. (2007). “Future approaches of nanomedicine in clinical science,” Medical Clinics of North America, 91: 963-1016
- Campbell S.C., Olson G.J., Clark T.R., McFeter G. (2001). Biogenic production of cyanide and its application to gold recovery. J. Ind. Microbiol. Biotechnol. 26, 134
- Damyanova S., Gomez L.M., Banares M.A., Fierro J.L.G. (2000). Thermal stability of titania-supported 12-Moybdophosphoric heteropoly compounds. Chem. Mater. 2000;12:501–510.
- Das S.K., Marsili E. (2010). “A green chemical approach for the synthesis of gold nanoparticles: characterization and mechanistic aspect,” Reviews in Environmental Science and Biotechnology.
- Durán N., Priscyla D. Marcato P.D., Oswaldo L. Alves, Gabriel IHDe Souza, Elisa Esposito (2005). Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. Journal of Nanobiotechnology, 3:8 1477
- Durán N., Marcato P.D., De Souza G.I.H., Oswaldo L. Alves, Elisa Esposito (2007). Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. Journal of Biomedical Nanotechnology Vol.3, 203–208
- Falkiewicz-Dulik M, Macura A.B. (2008). Nanosilver as substance biostabilising footwear materials in the foot mycosis prophylaxis. Mikologia Lekarska;15:145-150
- Fayaz M.A., Balaji K., Kalaichelvan P.T., Venkatesan R. (2009). “Fungal based synthesis of silver nanoparticles–an effect of temperature on the size of particles,” Colloids Surf B Biointerfaces, 74: 123- 126
- Gajbhiye M., Kesharwani J., Ingle A., Gade A., Rai M. (2009). Fungus-mediated synthesis of AgNPs and their activity against pathogenic fungi in combination with fluconazole. Nanomedicine: Nanotechnology, Biology, and Medicine, 5, 382–386
- Gratzel M. (2001). “Photoelectrochemical cells,” Nature, 414: 338-344
- Henglein A. (1993). J. Phys. Chem. 97, 547-5471
- Ingle A., Gade A., Pierrat S., Sönnichsen C., Rai M. (2008). Mycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria. Curr Nanosci; 4: 141-4
- Jaidev L.R., Narasimha G. (2010). Colloids Surf B. Biointerfaces. Dec 1; 81 (2): 430-3
- Jain J., Arora S., Rajwade J.M., Omray P., Khandelwal S., Paknikar K.M. (2009). Mol Pharm. Sep-Oct; 6 (5): 1388-401
- Kim J.S., Kuk E., Yu K.N., Kim J.H., Park S.J., Lee H.J. (2007). Antimicrobial effects of silver nanoparticles. Nanomedicine: NBM; 3:95-101
- Kim K.J., Sung W.S., Moon S.K., Choi J.S., Kim J.G., Dong G.L. (2008). Antifungal effect of AgNPs on dermatophytes. J Microbiol Biotechnol; 18: 1482-4
- Kim K.J., Sung W.S., Suh B.K., Moon S.K., Choi J.S., Kim J.G. (2009). Antifungal activity and mode of action of silver nano-particles on Candida albicans. Biometals;22:235-42
- Labrenz M., Druschel G.K., Thomsen E.T., Gilbert B., Welch S.A., Kemner K.M. (2000). Formation of sphalerite (ZnS) deposits in natural biofilms of sulfate-reducing bacteria. Science; 290:1744-7
- Lee H.J., Y eo S.Y., Jeong S.H. (2003). Antibacterial effect of nanosized silver colloidal solution on textile fabrics. J. Mater. Sci. 38, 2199
- Lee H.J., Jeon S.H. (2004). Bacteriostasis of nanosized colloidal silver on polyester nonwovens. Text. Res. J. 74, 442
- Magudapathy P., Gangopadhyay P., Panigrahi B.K., Nair K.G.M., Dhara S. (2001). Physics B. 299, 142-146
- Mandal D., Molander M.E., Mukhopadhyay D., Sarkar G., Mukherjee P. (2006). “The use of microorganism for the formation of metal nanoparticles and their application,” Applied Microbiology and Biotechnology, 69: 485-492
- Mansoori G. A. (2005). World Scientific Pub. Co., Hackensack, N.J.
- Marcato P.D., De Souza G.I.H., Alves O.L., Esposito E., Durán N. (2005). Antibacterial activity of silver nanoparticles synthesized by Fusarium oxysporum strain. 2nd Mercosur Congress on Chemical Engineering 4th Mercosur Congress on Process Systems Engineering
- Mukherjee P., Ahmad A., Mandal D., Senapati S., Sainkar S.R., Khan M.I., Ramani R., Parischa R., Ajaykumar P.V., Alam M., Sastry M., Kumar R. (2001A). Angew. Chem., Int. Ed. Engl., 40, 3585–3588
- Mukherjee P., Ahmad A., Mandal D., Senapati S., Sainkar S.R., Khan M.I., Parishcha R., Ajaykumar P.V., Alam M., Kumar R., Sastry M. (2001B). Fungus-mediated synthesis of AgNPs and their immobilization in the mycelial matrix: A novel biological approach to nanoparticle synthesis. Nano Letters, 1 (10), 515–519
- Narayanan K.B., Sakthivel N. (2010). Advances in Colloid and Interface Science 156, 1–13
- Pal S., Tak Y.K., Song J.M. (2007). Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol; 73:1712-20
- Panáčeka A., Kolářb M., Večeřováb R., Pruceka R., Soukupováa J., Kryštofc V., Sanghi R., Verma P. (2009). Biores. Technol., 100, 501-504
- Petica A., Gavriliu S., Lungua M., Burunteaa N., Panzarub C. (2008). Colloidal silver solutions with antimicrobial properties. Mater Sci Eng B; 152:22-7
- Ray S., Sarkar S., Kundu S. (2011). Extracellular biosynthesis of AgNPs using the mycorrhizal mushroom Tricholoma crassum (BERK.) SACC: Its antimicrobial activity against pathogenic bacteria and fungus, including multidrug resistant plant and human bacteria. Digest Journal of Nanomaterials and Biostructures, Vol. 6, No 3, July – September, p.1289-1299
- Roh Y., Bai J., Lauf R.J, Mcmillan A.D., Phelps T.J., Rawn C.J. (2001). Microbial synthesis of metal-substituted magnetites. Solid State Commun; 118: 529-34
- Saha S., Sarkar J., Chattopadhyay D., Patra S., Chakraborty A., Acharya K. (2010). Production of AgNPs by a phytopathogenic fungus Bipolaris nodulosa and its antimicrobial activity. Digest Journal of Nanomaterials and Biostructures Vol. 5, No 4, October-December, p. 887-895
- Saha S., Chattopadhyay D., Acharya K. (2011). Preparation of silver nanoparticles by bio-reduction using Nigrospora oryzae culture filtrate and its antimicrobial activity. Digest Journal of Nanomaterials and Biostructures Vol. 6, No 4, October-December, p. 1519-1528
- Sanghi R., Verma P. (2009). Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresource Technology, Volume 100, Issue 1, January, Pages 501–504
- Sastry M., Patil V., Sainkar S.R. (1998). Electrostatically controlled diffusion of carboxylic acid derivatized silver colloidal particles in thermally evaporated fatty amine films. J Phys Chem B, 102:1404-1410
- Sastry M., Ahmad A., Khan M.I., Kumar R. (2003). “Biosynthesis of metal nanoparticles using fungi and actinomycete,” Current Science, 85,2: 162-170
- Shahverdi A.R., Fakhimi A., Shahverdi H.R., Minanian S. (2007A). Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against S. aureus and E. coli. Nanomedicine: NBM; 3:168-71
- Shahverdi A.R., Minaeian S., Shahverdi H.R., Jamalifar H., Nohi A. (2007B). “Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria: A novel biological approach,” Process Biochemistry, 42: 919-923
- Shankar S.S., Ahmad A., Parischa R., Sastry M. (2003). “Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes,” Journal of Material Chemistry, 13: 1822-1826
- Shankar S.S., Raj A., Ankamwar B., Singh S., Ahmad A., Sastry M. (2004). Biological synthesis of triangular gold nanoprisms. Nat. Mater, 3, 482
- Silver S., Phung L.T. (1996). Anal. Rev. Microbiol. 50, 753-789
- Singh M., Manikandam S., Kumaraguru A.K. (2011). Research Journal of Nanoscience and Nanotechnology, 1(1): 1-11
- Slawson R.M., Van Dyke M.I., Lee H., Trevors J.T. (1992). Germanium and silver resistance, accumulation and toxicity in microorganisms, Plasmid, 27, p 72–79
- Smith A.D., Hunt R.J. (1985). Solubilization of gold by Chromobacterium violaceum J. Chem. Technol. Biotechnol. 35B, 110 15
- Sondi I., Salopek-Sondi B. (2004). J. Colld. Interf. Sci. 275, 177
- Spadaro J.A., Berger T.J., Barranco S.D., Chapin S.E., Becker R.O. (1974). Antibacterial effects of silver electrodes with weak direct current. Antimicrob. Agents Chemother., 6, p 637–642
- Stoimenov P.K., Klinger R.L., Marchin G.L., Klabunde K.J. (2002). Metal oxide nanoparticles as bactericidal agents. Langmuir, 18, p 6679–6686
- Vahabi K., Mansoori A.G., Karimi S. (2011). Biosynthesis of AgNPs by fungus Trichoderma Reesei (A Route for Large-Scale Production of AgNPs). Insciences J., 1(1), 65-79
- Xia Y., Yang P., Sun Y., Wu Y., Mayers B., Gates Y., Yin Y., Yan H. (2003). “One-dimensional nanostructures: synthesis, characterization and applications,” Advanced Materials, 15: 353-389
- Y eo S.Y., Jeong S.H. (2003). Preparation and characterization of polypropylene/ silver nanocomposite fibers. Polymer Intern. 52, 1053
- Y eo S.Y., Lee H.J., Jeong S.H. (2003). Preparation of nanocomposite fibers for permanent antibacterial effect. J. Mater. Sci. 38, 2143
- Zhao G.J., Stevens S.E. (1998). Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion, Biometals, , 11, p 27–32.

This work is licensed under a Creative Commons Attribution 4.0 International License.





