How to Cite | Publication History | PlumX Article Matrix
A. Amini et al.
1Department of Laboratory Sciences, School of Paramedical, Golestan University of Medical Sciences, Gorgan, Iran. 2Laboratory Science Research Center, Department of Laboratory Sciences, School of Paramedical, Golestan University of Medical Sciences, Gorgan, Iran. 3Department of Microbiology, School ofMedicine, Golestan University of Medical Sciences, Gorgan, Iran 4Laboratory of Tuberculosis, Health Center of Golestan Province, Golestan University of Medical Sciences, Gorgan, Iran. 5Young Researchers Club, Lahijan Branch, Islamic Azad University, Lahijan, Iran. 6Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran.
DOI : http://dx.doi.org/10.13005/bbra/1095
ABSTRACT: The mec A gene in Staphylococcus aureus leads to production of new penicillin-binding protein called PBP2a.This change may follow some changes in other phenotypes. The aim of this study was the comparison of Ferment Sugars, Produce Hemolysis and Measuring Growth in MRSA and MSSA isolates.188 Staphylococcus aureus isolates separated from inpatients and healthcare workers (healthy carriers)were studied.Bacterialcultures in blood agar environment at 37ºC during 24h and at 4ºC during other 24h were applied for studying hemolysis. Sugar fermentation carried out in phenol red Broth medium, containing glucose, galactose, arabinose, fructose, xylose, ramnose, mannose, sucrose, trehalose, raffinose or maltose. For determining bacterial growth,bacterial concentration of 103was taken each hour during 12 cultured in MHAand colonies were counted after 24h.The mean amount of hemolysis diameter in MRSA isolates was rather more than that of MSSA isolates. The difference between MRSA and MSSA isolates were significant as to fermenting ramnose, trehalose, galactose and xylose. The mean rate of growth in MRSAwere significantly different from that of MSSAisolates (p<0.05).Resistance to methicillin in Staphylococcus aureus isolates accompanies the increase of ability to ferment sugars. This phenomenon may be one of reasons for increased pathogenicity of MRSA isolates; So results shows the logarithmic phase is longer in MRSA isolates, This may implicate that PBP2a production in methicillin-resistant isolates follows slowing down nutrients entrance into the bacterium that in turn may causes slow growth .
KEYWORDS: Staphylococcus aureus; MRSA; MSSA; Sugar Fermentation; Hemolysis; Generation Time
Download this article as:| Copy the following to cite this article: Amini A. Comparison of Ferment Sugars, Produce Hemolysis and Measuring Growth in Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus Isolates from Inpatients and Healthcare Workers in Gorgan Hospitals, North of Iran. Biosci Biotechnol Res Asia 2013;10(1) |
| Copy the following to cite this URL: Amini A. Comparison of Ferment Sugars, Produce Hemolysis and Measuring Growth in Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus Isolates from Inpatients and Healthcare Workers in Gorgan Hospitals, North of Iran. Biosci Biotechnol Res Asia 2013;10(1). Available from:https://www.biotech-asia.org/?p=9927 |
Introduction
Staphylococcus aureus is one of factors causing hospital and community-acquired infections. It can result in some important diseases such as bacteremia, endocarditis, osteomalacia, toxic shock syndrome and dermaticinfections (26). This bacterium lives generally on anteronasal section. About 20-40% of healthy ones can be carriers of the Staphylococcus aureus at any time. The possibility of being the carrier of this bacterium increases in some situations such as healthcare workers in hospitals;then the infection can transmit to others, especially patients, by hospital personnel (17). This microorganism is the leader of agentsresulting in bacteremia, surgical wounds,dermatic, soft tissueinfections, and sometimes mortality (3). The main features of the bacterium are its ability to cause disease, produce toxins and enzymes, and acquire resistance to antibiotics (22).The discovery of methicillin resistant isolates during the 1960s increased the importance of this bacterium (23). The mechanism of resistance to methicillin involves mainly producing anew changed PBP (Penicillin Binding Protein) named PBP2a in bacterium cell wall which has a weak tendency to bind beta-lactam drugs and is not inhibited by these drugs (8). Resistance to methicillin is coded by mecA gene which is located on SCCmec element. SCCmec is a mobile genetic element that can be transmitted among different staphylococcus species (21).
Change in PBP may result in some change in bacterium wall structurewhich in turn accompanies other changes in some phenotypes. These changes have been well documented in related literature. It has been proved that the rate of enterotoxin B produced by Staphylococcusaureus is related to its resistance to methicillin (25).Studies on the presence of leukocidin panton valentine genes showed some differences between MRSA and MSSA isolates (7, 18). In comparison with MSSA cells, MRSA cells have more lipids of all kinds (19). Experiments showed that MSSA isolates have shorter generation time than MRSA isolates; Then, the former produce more cells per hour than the latter. In other words, growth logarithmic phase in the same conditions is longer in MRSAthan MSSA isolates and it is possible that the time for the division of methicillin-resistant cellswould be longer (20). Several clinical studies revealed that the costs, treatment duration, pathogenesis and mortality rate are higher in MRSA than MSSA (21). The possible differences in pathogenesis and virulence between MRSA and MSSA are ever an open question.
Different views on the pathogenic role of MRSA have encouraged many researchers to investigate the role-playing factors in pathogenesis of MRSA and MSSA isolates. The current comparative study aimed to investigate the ability of methicillin-resistant and methicillin-sensitive Staphylococcus aureus isolates to fermentsugars, hemolysis production in blood agar, and measure bacterial growth rate.
Material and methods
This cross-sectional descriptive study was conducted on 188 isolates ofStaphylococcus aureusseparated from inpatients (0.59%) and healthcare workers (healthy carriers)(0.41%) in hospitals located in Gorgan city, Iran, during 2010-11.
Identification of Bacteria
Standard methods were used for identifying Staphylococcus aureus including gram staining, culturing in Mannitol Salt Agar (MSA), catalase, DNase and coagulase in forms of slide and tube procedures (9, 24).
Polymerase Chain Reaction (PCR)
MRSA isolates were identified by PCR method as a standard test for detecting the existence or lack of methicillin-resistant gene (mec A). This was done by paired primers5′- AAATCAGATGGTAAAGGTTGGC -3′ and 5′- AGTTCTGCAGTACCGGATTTGC -3′(14).
Sugars Fermentation
For testing sugar fermentation,Sugars thatStaphylococcus aureus can or can’t ferment based on diagnostic tables was used (11). The selected sugar solutions of 1%includingglucose, galactose, arabinose, fructose, xylose, ramnose, mannose, sucrose, trehalose, raffinose or maltose were added to tube containing 5 ml of phenol red brothmedium (3). Bacterium suspension of 100ml equal to concentration of 0.5 McFarland from 24h culture was added to each sugarcontained phenol red broth (4). All media were maintained under 37ºCduring 24 h. The stain change of medium from red to yellow shows the sugar fermentation.As all Staphylococcus aureusisolates consume glucose, the stain changes for this sugar were observed and recorded in three times: 4h, 8h and 24h (11).
Produce Hemolysis
Test for hemolysis in Blood Agar (BA) medium was done for each Staphylococcus aureus isolate. First, sinks with of 5mm diameter were on blood agar culture. Second, bacterium suspension of 80 ml equal to the concentration of 0.5 McFarland was added to each sink. All media were incubated in 37ºC for 24 h. Then, the hemolysis production and its diameter were observed. In order to observe cold-hot hemolysin, they were maintained in freezer and after 24 h, the number and diameter of hemolysisproduced in MRSA and MSSA isolates were observed again and compared (4).
Measuring Growth Rate
For determining growth rate, turbidity of 0.5 McFarland equivalent was diluted as serial dilution until its ultimate concentration reached 103 CFU/ml (indicator tube);this was considered as zero hour of bacterial growth.Tube was incubated during 12 h in 37ºC. Sampling, observing by graded slide and culturing in Mueller-Hinton Agar (MHA) were done each 2-3 h period. For this, bacterium suspension of 10µl was distributed on MHA culture. Graded slide was applied for counting bacteria. Counted number was multiplied in inverse dilution coefficient for determining the number of bacteria in each ml of broth medium. Samples were diluted after fourth hour for counting bacteria. In the current study, plates with colony count in the range of 30-300 CFU/ml were considered as countable (10, 28). After repeating the experiment, it was appeared that the best times for measuring bacterial logarithmic growth were between 4th -10thh and the growth enters stable phase after 10 h. Then, growth between 4-10 h was measured as growth rate.
Results
PCR
Based on PCR method, 61 (32.4%) and 127 (67.6%) out of all 188 isolates of Staphylococcus aureus were resistance and sensitive to methicillin, respectively.
Sugars Fermentation
Glucose was fermented by all Staphylococcus aureus isolates during 24 h; that in 10 (16.4%) isolates fermentation was observable after 4thh, but in MSSA isolates, only 8 (6.3%) isolates fermented the sugar (P<0.05). More than 94% of Staphylococcus aureus isolates (178<) fermented mannose, sucrose, fructose, terhalose and maltose; and 86.2% of isolates (= 162) fermented galactose. As table 1 shows, differences in fermentation for MRSA and MSSA were significant as to ramnose[11 (18%) and 3 (2.4%), respectively, (p<0.001)], xylose[7 (11.5%) and 3 (2.4%), respectively, p=0.01], galactose [57 (93.4%) and 105 (82.7%), respectively, p=0.03] and terhalose [55 (90.2%) and 126 (99.2%),respectively, p<0.005].
Produce Hemolysis
64 (34%) of Staphylococcus aureus isolates could not create hemolysis in 24 h. After placing the plates in cold situation for other 24h (48 h culture), the amount decreased in 50 (26.6%) isolates;this demonstrates a 7.4% increase in hemolysis production in cold situation.
The number of hemolysis layers around the bacterium in 24 h culture was more than those of 48h culture. In 48 h culture, 50 isolates had 3 or 4 hemolysis layer. In 24h culture, however, no isolate had more than 3 hemolysis layers (Figure 1 and Table 2). This difference was significant (p<0.001). The mean amounts of hemolysis diameter in 24h culture (9.7ml) and 48hculture (14.05) were statistically significant (p<0.001).
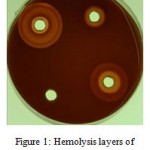 |
Figure 1: Hemolysis layers of Staphylococcus aureus.
|
Table 1: Comparing the abilities of MRSA and MSSA isolates to ferment sugars.
|
Ability Sugar |
Fermentation | P value | |||
| MRSA (%) | MSSA (%) | Total (%) | |||
| Glucose | (4 h) | 10 (16.4%) | 8 (6.3%) | 18 (9.6%) | 0.02 |
| (8 h) | 29 (47.5%) | 57 (44.9%) | 86 (45.7%) | 0.42 | |
| (24 h) | 61 (100%) | 127 (100%) | 188 (100%) | 1 | |
| Mannose | 59 (96.7%) | 125 (98.4%) | 184 (97.9%) | 0.39 | |
| Terhalose | 55 (90.2%) | 126 (99.2%) | 181 (96.3%) | 0.005 | |
| Galactose | 57 (93.4%) | 105 (82.7%) | 162 (86.2%) | 0.03 | |
| Maltose | 60 (98.4%) | 118 (92.9%) | 178 (94.7%) | 0.1 | |
| Fructose | 61 (100%) | 121 (95.3%) | 182 (96.8%) | 0.09 | |
| Xylose | 7 (11.5%) | 3 (2.4%) | 10 (5.3%) | 0.01 | |
| Arabinose | 8 (13.1%) | 12 (9.4%) | 20 (10.6%) | 0.29 | |
| Sucrose | 59 (96.7%) | 125 (98.4%) | 184 (97.9%) | 0.39 | |
| Ramnose | 11 (18%) | 3 (2.4%) | 14 (7.4%) | < 0.001 | |
| Raffinose | 17 (27.9%) | 24 (18.9%) | 41 (21.8%) | 0.11 | |
Table 2: Comparing the frequencies of hemolysis layers of Staphylococcus aureus in 24h and 48h cultures
| Hemolysis layers
Culture time |
0 (%) | 1 (%) | 2 (%) | 3 (%) | 4 (%) |
| 24 h (37 ºC) | 64 (34%) | 81 (43.1%) | 43 (22.9%) | 0* | 0* |
| 48 h (4 ºC) | 50 (26.6%) | 64 (34%) | 24 (12.8%) | 30 (16%) | 20 (10.6%) |
* No isolates in the two cultures had 3 or 4 hemolysis layers.
There was no significant difference between resistance or sensitivity to methicillin and hemolysis layers in 24h culture and 48h culture (p>0.05, Table 3).
Table 3: Comparing the frequencies of hemolysis layers in 24h and 48h cultures in MRSA and MSSA isolate
| Time & Layer
Isolates |
24 h (37 ºC) | 48 h (4 ºC) | ||||||
| 0 (%) | 1 (%) | 2 (%) | 0 (%) | 1 (%) | 2 (%) | 3 (%) | 4 (%) | |
| MRSA | 19(31.1%) | 27 (44.3%) | 15 (24.6%) | 10 (16.4%) | 22 (36.1%) | 11 (18%) | 12 (19.7%) | 6 (9.8%) |
| MSSA | 18 (35.4%) | 13 (42.5%) | 42 (22%) | 40 (31.5%) | 42 (33.1%) | 13 (10.2%) | 18 (14.2%) | 14 (11%) |
| Total | 64 (34%) | 81 (43.1%) | 43 (22.9%) | 50 (26.6%) | 64 (34%) | 24 (12.8%) | 30 (16%) | 20 (10.6%) |
Measuring Growth Rate
In this study, themean growth rate of Staphylococcus aureus isolates was 35.35 minutes (table 4).
Table 4: Descriptive statistic indicators for growth rate of Staphylococcus aureus isolates (in minutes)
| Mean | 35.35 |
| Median | 35.19 |
| Mode | 33.57 |
| SD | 3.84 |
ANOVA test revealed that the times of bacterial division in MRSA and MSSA were significantly different (p=0.01). So, the mean time for growth rate in methicillin-sensitive isolates and methicillin-resistant were35.05 and 36.32 minutes, respectively. Then logarithmic phase in MRSA isolates was longer (Table 5).
Table 5: Means and SDs in the studied groups(in minutes)
| Feature
Generation time |
MRSA | MSSA | Inpatient | Carrier |
| Mean | 36.32 | 35.05 | 36.07 | 34.45 |
| SD | 2.99 | 4.11 | 3.81 | 3.75 |
Discussion
The ability of Staphylococcus aureus to ferment sugar as main food sources is important. Most isolates of Staphylococcus aureus have the ability to produce acid from various sugar types, such as glucose, mannitol, mannose, terhalose, maltose and sucrose. This phenomenon was observed in isolates of our region (11). All isolates in our study fermented glucose and the process began in 10% of the isolates during the first 4h and increased in 46% during 8h;this demonstrates the intensive need of the bacterium to rapid consume this kind of sugar for growth. Since the current study showed that less than 10% of these isolates had the ability to ferment xylose and arabinose, some scientific resources such as Bergey’s Manual of Determinative Bacteriology (12) and The Prokaryotes(11) in their diagnostic tables showed the inability of Staphylococcus aureus to ferment these sugars. The ability to ferment raffinose by isolates in this region was reported 23% which is higher than expected amount (<10%) (3). However,Adegoke in 1982 (12) and Ajuwape in 2001 (2), reported that 81.7% and 46.3% Staphylococcus aureus isolates fermentedxylose, respectively.This difference may be the result of different features between domestic isolates and isolates in other regions; the deep study of the phenomenon in other regions of Iran can be effective in identifying Iranian domestic isolates. Also, it was identified that the ability of MRSA in fermenting each of above-mentioned sugars as well as ramnose was more than MSSA. It is proposed that further studies should be conducted for studying the role of PBP2a in acquiring such ability.
Studying 82 isolates of Staphylococcus aureusseparated from goat, Adegoke found that all isolates fermented glucose during 24h. In addition, 98.8% and 98.7% of the isolates fermented maltose and sucrose with acid production, respectively (1). All 108 isolates in Atuwape’s study fermented glucose, mannitol and sucrose and 98.1% and 89.1% of them fermented maltose and terhalose, respectively (2).The results of these two studies are In line with results achieved in our region.
Based on our findings, resistance to methicillin in Staphylococcus aureus isolates resulted in increasing their ability to fermentsugars, especially ramnose, xylose and galactose. The glucose fermentation in MRSA isolates was more than that of MSSA ones. Based on thereality, it can be hypothesized that PBP2a may increase the permeability of sugars into bacteria that needs further studies.
The hemolysins and leukocidins released by Staphylococcus aureus are of the famous systolic toxins. The bacterium produces4 different hemolytic toxins (α, β, γand δ), but different isolates produce different amounts of these toxins(27). In our study, 37% of Staphylococcus aureus isolates had not the ability to produce hemolysis in blood agar medium. In the study by Coia et al., out of 210 isolates, only 12 (6%) were unable to produce hemolysin. This amount is definitely lesser than that of our region (5).
Organisms that produce solution hemolysin in blood agar culture surface,create an area of beta hemolysis around their colonies (27). Alpha toxin is considered as a main factor for developing beta hemolysis area in blood agar at 37ºC. This toxin is produced by 85% of Staphylococcus aureus isolates (6). In Coia’s study, its frequency was reported around 60% and as a result,however, if this toxin is the main factor for hemolysis in blood agar, around 40% of isolates lack it (5), thatthis is relatively in line with our results. In a study in 2009, it was reported that alpha hemolysin coding gene (hla) in Staphylococcus aureus isolates of Austria, Hungary and Macedonia were 88%, 99.5% and 85%, respectively, that demonstrates the difference among hemolysin producing isolates in various European regions (16), but the amount of expressed and alpha hemolysin-producing genewas not reported. This exotoxin is a secretory protein with hemolytic, systolic, dermonecrotic and lethalfeatures.
Studies showed that the sensitivity of all cells to this toxin is not the same. For example, rabbit erythrocytes and human platelet have a high tendency to binding the toxin and some others such as human erythrocytes have a low tendency to do so (6). Then, when culturing Staphylococcus aureus samples, it is better to use sheep or rabbit blood rather than human blood. No use of human blood in some study settings may be a reason of hemolysis differences observed in previous studies.
In our study, the frequency of cold-hot hemolysin was analyzed by keeping the 24h bacterial culture in other 24h culture in freezer temperature and estimating the number of layers or diameters of hemolysis formed around bacterial colony. It was detected that 8.5% of isolates with no beta hemolysis in 37ºC, beta hemolysis was observed at freezer temperature and its diameter reached up to 8-15 mm and it had one or two layers. In addition, keeping in cold situation resulted in the increase of the amount of hemolysis layers and diameters of 66 isolates. It is estimated that there is a beta hemolysin in 43.1% isolates of Gorgan city, and Coia found the frequency of this hemolysin in Scotland was 21.9% (5). In accordance with our result, beta hemolysin coding gene (hib) of Staphylococcus aureus isolates in Macedonia and Hungary was about 33-62% (16).
In line with our results, Coia found no significant differences between MRSA and MSSA isolates as to beta hemolysin production and the number of the producers of this hemolysin (14 out of 49 vs. 30 out of 152) (p>0.05) (5). However, in the study by Kocsis in each three studied countries, the frequencies of beta hemolysin producing gene in the two isolate groups were significantly different. It is notable that beta hemolysin in MRSA isolates belonged to Macedonia and Hungary was higher, but the situation was the reverse in Austria case (16). A possible reason for the difference between the results of this study and those of our study as well as Coia’s is that we tested the phenotype and the two studies tested the genotype. Considering the results of the study, it is possible that some isolates in some regions contain more gene of this kind than isolates in other ones.
Beta toxin is a molecule with molecular weight of 35 KD and has Mg2+Sphingomyelinase (SMase) activity. As a result, the sensitivity of host cells depends on membrane SMase rate. This toxin is produced by many Staphylococcus aureus isolates and well possiblydestroys tissue and causes abscess along with alpha toxin. Erythrocytes of sheep, goat and cow have more sensitivity to this toxin. As having the highest amount of SMase in outer row of the membrane of their two cytoplasm layers, leucocytes are the most sensitive cells to this toxin. Beta toxin has a known role in the inflammation of cow breast. Aarestrup’s study showed that beta toxin was produce in 72% of isolates from cow breast, 11% of isolates from healthy carriers and 13% of isolates from human septicemia (13).
The frequency of hemolysin producing MRSA and MSSA isolates were compared in the current study. No significant difference was observed between the two isolate groups as to hemolysis diameter and number. The hemolysin lacking MSSA isolates, however, were more than hemolysin lacking MRSA isolates (30% vs.17%); this demonstrates that methicillin resistant isolates have more tendencies to producing hemolysin. Coia found no significant difference between MRSA and MSSA isolates in producing alpha hemolysin (p>0.05). However, MRSA isolates produced higher rate of this toxin than MSSA isolates (36 out of 49 vs.86 out of 152). In this study, 12 isolates (7 MSSA and 5 MRSA) out of 210 ones were able to produce hemolysin. In addition, the rate of hemolysin production, especially multiple one was higher in MRSA than MSSA isolates (5). Kocsis et al., however, reported that the frequencies of alpha hemolysin producing gene between these two isolate groups were not significantly different in Austria and Hungary cases. But the situation was the reverse in Macedonia case, in which the difference was significant and 96% and 72% of methicillin-resistant and methicillin-sensitive isolates had this hemolysin, respectively. In the three studied countries, the frequencies of producing alpha hemolysin in methicillin-resistant were higher than that of methicillin-sensitive isolates (p<0.001) (16);this may be a cue for the fact that MRSA isolates are more pathogenic than MSSA isolates.
As Domingue reported, Staphylococcus aureus has two cell division times in logarithmic phase; one relates to in-vivo condition lasting 60 minutes and the other relates to in-vitro condition lasting about 24 minutes (15). Based on ‘Prescott’s Microbiology’ book, the growth ofStaphylococcus aureus is 28.2 minutes. In another study, this rate is reported between 27-30 minutes (28). In our study, however,the generation time of the bacterium was 35.3 minutes.
Several experiments showed that the generation time of MSSA is shorter than that of MRSA isolates and consequently, the former produce more cells than the latter per hour. In other words, logarithmic phase of growth in the same conditions are longer in MRSA isolates than MSSA ones. It is possible that the time of cell division in methicillin-resistant isolates is longer (20). Based on our results and in line with the above mentioned study, the bacterial division times of MRSA and MSSA isolates are different. The mean±SD of generation times in methicillin sensitive and methicillin resistant isolates were 35±4.11 and 36.3±2.99, respectively;this shows that the logarithmic phase of MRSA is longer than that of MSSA isolates.Karauzum reported the cell division time of MRSA and MSSA isolates 31±2.67 and 30.5±1.35, respectively (15). The result does not accord ours as to generation time, and cell division time in methicillin-resistant was longer than methicillin-sensitive isolates. This fact may implicate that PBP2a production in methicillin-resistant isolates accompanies some change in nutrients’ permeability into the bacterium. Slowing down nutrients’ entrance into the bacterium causes slow growth. As the ability of many studied sugars to be fermented by MRSA is higher than that of methicillin-sensitive isolates, other factors, such as slowing aminoacid, and vitamin entrance appear to involve in slowing down the growth of this bacterium. It is necessary to design studies for comparing the entrance and use of these substances in MRSA and MSSA isolates.
References
- Adegoke, G.O., Ojo, M.O. Biochemical Characterization of Staphylococci isolated from Goats. Vet. Microbiol.1982; 7: 463-470.
- Ajuwape, A.T., Aregbesola, E.A. Biochemical Characterization of Staphylococcus isolated from Rabbits. Isr. J.Vet. Med.2001; 56: 17-21.
- Atlas, R.M. (ed):Handbook of Microbiological Media,4nd edn. Boca Raton: Fla, C.R.C. Press,2010.
- Benson, J.H. (ed):Microbiological Applications: A Laboratory Manual in General Microbiology, 8nd edn. New York: McGraw-Hill Book Press, 2001.
- Coia, J.E., Browning, L., Haines, L., Birbeck, T.H., Platt, D.J. Comparison of Enterotoxins and Haemolysins Produced byMethicillin-Resistant (MRSA) and Sensitive (MSSA)Staphylococcus aureus.J. Med. Microbiol.1992;36: 164-171.
- Dinges, M.M., Orwin,P.M., Schlievert, P.M. 2000. Exotoxins of Staphylococcus aureus.Clin. Microbiol. Rev. 2000;13: 16-34.
- Diep, B.A., Carleton, H.A., Chang, R.F., Sensabaugh, G.F., Perdreau- Remington, F. Roles of 34 Virulence Genes in the Evolution of Hospital- and Community- Associated Strains of Methicillin-Resistant Staphylococcus aureus. J. Infect. Dis. 2006;193: 1495-1503.
- 8.Finan, J.E., Rosato, A.E., Dickinson, T.M., Ko, D.,Archer, G.L. Conversion of Oxacillin-Resistant Staphylococci from Heterotypic to Homotypic Resistance Expression.Antimicrob. Agents. Chemother. 2002; 46: 24-30.
- Forbes, B.A., Sahm, D.F., Weissfeld, A.S. (ed):Bailey&scotts Diagnostic Microbiology, 12nd edn. USA: Elsevier, 2007.
- Bitton, G. (ed): Microbial Metabolism and Growth. In Wastewater Microbiology, 3nd edn. Wiley-Liss Press: New Jersey,2005; pp 45-74.
- Gotz, F., Bannermant, T., Schleifer, K. The Genera Staphylococcus and Macrococcus. InThe Prokaryotes, Volume 4, 3nd edn. M.Dworkin., S. Falkow., E. Rosenberg(ed).Springer Press:New York.2006; pp 61-70.
- Schleifer, K.H., Bell, J.A. Staphylococcaceae Family. In Bergey’s Manual of SystematicBacteriology, Volume 3: The Firmicutes.P.D. Vos., G. Garrity., D. Jones., N.R. Krieg., W. Ludwing., F.A. Rainey and et al (ed). Springer Press: New York. 2009; pp 392-426.
- Huseby, M., Shi, K., Brown, C.K., Digre, J., Mengistu, F., Seo, K.S.,and et al. Structure and Biological Activities of Beta Toxin from Staphylococcus aureus.J. Bacteriol.2007; 189: 8719-8726.
- Japoni, A., Alborzi, A., Rasouli, M., Pourabbas, B. Modified DNA Extraction for Rapid PCR Detection of Methicillin-Resistant Staphylococci. Iranian. Biomedical. J. 2004;8: 161-165.
- Karauzum, H., Ferry, T., Bentzmann, S., Lina, G., Bes, M., Vandenesch, F.,and et al. Comparison of Adhesion and Virulence of Two Predominant Hospital-Acquired Methicillin-Resistant Staphylococcus aureus Clones and Clonal Methicillin-Susceptible S. aureusisolates. Infect. Immun.2008; 76: 5133-5138.
- Kocsis, E., Lagler, H., Pesti, N., Stich, K., Kristof, K., Nagy, K., and et al. Comparison of Austrian, Hungarian and Macedonian Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureusStrains in Relation to Prevalence of Cytotoxin Genes. Microb. Pathog.2009; 46: 328-336.
- Lowy, F.D. Staphylococcal Infections. InHarrison’s Principles of Internal Medicine, 17nd edn. E. Braunwald,.D.L. Kasper., S.L. Hauser., D.L. Longo., J.L. Jameson., and et al (ed). McGraw-Hill Book Press:New York. 2008; pp. 872-880.
- Robert, J., Etienne, J., Bertrand, X. Methicillin-Resistant Staphylococcus aureusProducing Panton–Valentine Leukocidin in a Retrospective Case Series from 12 French Hospital Laboratories, 2000-2003.Clin. Microbiol. Infect.2005; 11: 585-587.
- Rozgonyi, F., Kiss, J., Levai, G.,Biacs, P.,Vaczi, L. Phenotypic Expression of Methicillin Resistance in Staphylococcus aureus. InPlasmids, medical and theoretical aspects. S. Mitsuhashi., L. Rosival,. V. Krcmery (ed). Springer Verlag Press: New York.1977; pp 353-360.
- Rozgonyi, F., Laczko, J.,Vaczi, L. Phenotypic Expression of Resistance to Penicillinase-Stable Beta-Lactams in Staphylococcus aureus: Growth Rate, Cross Wall Morphogenesis and Cell Division Cycle. FEMS. Microbiology. Letters.1982; 14: 237-240.
- Rozgonyi, F., Kocsis, E., Kristof, K., Nagy, K. Is MRSA More Virulent than MSSA? Clin. Microbiol. Infect. 2008; 13: 843-845.
- Amini, R., Abdulamir, A.S., Ling, B.P., Jahanshiri, F., Hematian, A., Zargar, M., et al. Isolation and Identification of Methicillin- Resistant Staphylococcus aureus from Keys of College Students Using Different Detection Methods. British. Biotech. J. 2012; 2: 13-25.
- Shurland, S., Zhan, M., Bradham, D., Roghmann, M.C. Comparison of Mortality Risk Associated with Bacteremia Due to Methicillin-Resistant and Methicillin-Susceptible Staphylococcus aureus. Infect. Control. Hosp. Epidemiol. 2007;28: 273-279.
- Vaez, H., Ghazi Saeidi, K., Moradi, A., Tabaraei, A., Khodabakhshi, B., Bazouri, M., and et al. Antibiotic Resistance Pattern of Methicillin Resistant Staphylococcus aureus isolated from Health-Educational Centers of Gorgan, Iran, 2008-2009. Iranian. J. Medical. Microbiology.2009; 3: 31-36.
- Waldrogel, F.A.Staphylococcus aureus. InPrinciples and Practice of Infectious Diseases, 7nd edn. G.L. Mandell., J.E. Bennett., and R. Dolin. Churchill Livingstone Press:Philadelphia.2009; pp 2543-2578.
- Weichhart, T., Horky, M., Sollner, J., Gangl, S., Henics, T., Nagy, E., and et al. Functional Selection of Vaccine Candidate Peptides from Staphylococcus aureus Whole-Genome Expression Libraries in Vitro. Infect. Immun.2003; 71: 4633-4641.
- Willet, H.P. Antimicrobial agents. InZinsser Microbiology, 20ndedn. W.K. Joklik., H.P. Willett., D.B. Amos., C.M. Wilfert (ed). Appleton & Lange,Norwalk: CT.1992; pp 401-416.
- Willey, J., Sherwood, L.,Woolverton, C. (ed): Microbial Nutrition, Growth and Control. In Prescott’s Microbiology, 7nd edn. McGraw-Hill Higher Education Press, New York. 2008; pp 119-148.

This work is licensed under a Creative Commons Attribution 4.0 International License.





