How to Cite | Publication History | PlumX Article Matrix
Abbas M. Al-Azab1, Khalid M. Al-Ghamdi2*, Mohamed A. Shaheen3 and Ahmed A. Zaituon4
1,3,4Department of Arid land Agriculture, Collage of Meteorology, Environment and Arid land Agriculture, 2Department of Biological Sciences - King Abdulaziz University, Saudi Arabia.
DOI : http://dx.doi.org/10.13005/bbra/1097
ABSTRACT: Protein samples of (larva, pupa and adult) for lab and field strains of dengue fever vector, Ae. aegypti were isolated. Proteins were analyzed using sodium dodecyl sulfate polyacrylamide (SDS-PAGE).The SDS-PAGE analysis showed that, total of 6 protein bands were detected. The relative molecular weight of the detected bands was approximately in the range of 16.6 – 75.6 kDa. The 16.6 kDa protein band was observed through all insect stages. In contrary, protein bands corresponding to (19.1 ,41.7 and 75.6 kDa) were identified specifically in adults of field strain whereas, the lab strain adults revealed specific protein band of 36 kDa. In the meantime, pupa protein pattern exhibiting four protein bands with molecular weight of 16.6, 46.8 , 69 and 75.6kDa The obtained results provide basic information would be utilized for the identification of differentially specific protein as a target oriented for specific agent of Ae. aegypti. Further studies are needed to identify these protein and their biological roles in dengue fever infections to be implemented in integrated insect pest management.
KEYWORDS: Dengue fever; Aedes aegypti; protein profiles; SDS-PAGE
Download this article as:| Copy the following to cite this article: Al-Azab A. M, Al-Ghamdi K. M, Shaheen M. A, Zaituon A. A. Protein Analysis of Dengue Fever Vector Aedes Aegypti, Using SDS-PAGE in Jeddah Governorate-Saudi Arabia. Biosci Biotechnol Res Asia 2013;10(1) |
| Copy the following to cite this URL: Al-Azab A. M, Al-Ghamdi K. M, Shaheen M. A, Zaituon A. A. Protein Analysis of Dengue Fever Vector Aedes Aegypti, Using SDS-PAGE in Jeddah Governorate-Saudi Arabia. Biosci Biotechnol Res Asia 2013;10(1). Available from:https://www.biotech-asia.org/?p=10165 |
Introduction
Mosquitoes are still the world’s number one vectors of human and animal, transmit a broad range parasitic and viral diseases. Dengue fever is now endemic in more than hundred countries in Africa, Mediterranean region, South America and South East Asia (WHO ,200,2003 ,Whitehorn and Farrar, 2010).). Until the 1960s, systematic data were generally collected from morphological and behavioral variations. However, after the 1960s biological macromolecules gained an increasingly important role in evolutionary and systematic studies. Early molecular studies with systematic purposes were concerned largely with proteins. The electrophoresis of proteins is an effective technique for generating systematic data from macromolecules. This method has become increasingly popular among systematists (Onaric and Sumer, 2003).
Electrophoresis has been proven useful in insect taxonomy where conventional taxonomic methods of identification of immature stages of related species have been inadequate (Berlocher, 1980). In the last 20 years, electrophoresis has been widely used in population genetics studies of more than 1100 species of animals and plants (Nevo et al., 1984). The electrophoresis of proteins is an effective technique for generating systematic data from macromolecules.
Gel electrophoresis technique of proteins is the most widely use molecular technique in insect systematic. This technique relies on the fact that identical proteins migrate the same distance under the electrical force applied to an electrophoretic gel while non-identical proteins usually migrate different distances. The proteins used in insect systematics are usually enzymes (Sheppard and Smith 2000). An accurate identification of the species of mosquitoes is required to determine whether it belongs to a species group that poses a potential risk (Gupta and Preet 2012). Recently, several studies have been isolated and described total , specific protein and gene expression from mosquitoes such as Aedes, Culex and Anopheles from immature stages and specific parts of adult antenna, salivary glands and protein bindings with dengue virus (Ishida et al ., 2002, Chee and AbuBakar 2004 , Hung et al. 2004 , Rohani, et al ., 2005 and Popova-Butler and Dean 2008 )
The present study conducted to identify the total protein profile of different developmental stages of Ae.aegypti dengue fever vector using SDS-PAGE analysis.
Materials and methods
Study sites
The presented investigation was conducted using Ae. aegypti collected from 5 sites of Jeddah provinces in western Saudi Arabia(Table1). The dengue cases and the re-epidemics were continuously reported in the selected location during the past six years.
Table 1: Coordinate of study locations in Jeddah governorate.
|
Location |
Coordinate | |
| E | N | |
| A | 39.187563 | 21.48253853 |
| B | 39.20689261 | 21.4728754 |
| C | 39.20259096 | 21.45213269 |
| D | 39.25174507 | 21.47087433 |
| E | 39.2111804 | 21.58961988 |
Where:
A= Al-Balad
B= Al-Nazlah Al-Yamaneyyah
C= Ghuleel
D= Al-Jameiah
E= Al-Safa
Collection and identification of mosquito
Adults of Ae. aegypti were collected from five different localities of Jeddah governorate. Adults and larvae stages were identified by using standard taxonomic keys ( Wood et al. , 1979 and Darsie and Ward, 2005 ). Briefly Ae. aegypti adults can be identified throughout the white scales on the dorsal (top) surface of the thorax that form the shape of a violin or lyre. The larvae stages can be identified using the comb scales on the eighth segment of the abdomen and the shape of the pectin teeth on the siphon . In larvae, the comb teeth have well developed lateral dentiles but the pectin teeth have less defined denticles.
Mosquito
Ae. aegypti (L.) larvae were reared under the laboratory conditions for several generations . Adults, larvae and pupae were used to isolate total protein in this study. A field strain of Ae. aegypti larvae were collected from Jeddah governorate. This stock colony was maintained at a room temperature at 27±l°C and 70±5% R.H., with a 14:10 (L: D) photoperiod.
Mosquito Total Protein Electrophoresis
Sodium dodecylsulfate polyacrylamide gel electrophoresis was used to identify Ae. aegypti by their protein analysis according to the method of Laemmli 1970 , and modified by Studier (1973).
Protein extraction
Adults or immature stages of Ae. aegypti were extracted according to Abo-El-Saad. and Ajllan. (2003). Mosquito stages were finely homogenized together in eppendorf tubes containing 200 µl of the extraction buffer by a handle plastic homogenizer and left in refrigerator at 4°C over night then vortex for 15 seconds and centrifuged at 12,000 rpm at 4°C for 15 minutes. The supernatants were transferred to new eppendorf tubes and kept in deep – freezer until use for electrophoretic analysis.
Preparation of protein samples
A 20 µl of the samples as extracted previously from sample with 5 µl of 2 % SDS-sample buffer. The mixture was incubated in a boiling water bath for 5 min before application.
Application of protein samples
The protein samples of 25 µg protein were loaded into the wells using a Hamilton syringe. Protein fractionated by SDS polyacrylamide gel electrophoresis (PAGE) as described by Smith (1976); using slab gel that consist of a 4% polyacrylamide stacking gel and an acrylamide (12%) gel. High and low MW standards were used for the determination of protein profiles of all fractionated samples.
Electrophoretic analysis
Protein fractionated by SDS polyacrylamide gel electrophoresis (PAGE) as described by Smith (1976). High and low MW standards were used for the determination of protein profiles of all fractionated samples.
Results and discussion
The protein samples were purified from the larva, pupa and adults(lab and field stain) stages. The protein banding of lab and field strain adult and immature stages of dengue fever vector, were electrophoretic. The Coomassie-stained SDS-PAGE was applied to analysis the purified protein sample of the different insect stages. Two gels were obtained for different developmental stages of Ae.aegypti as shown in Fig.(1 and 2).The protein purified samples were quantified using bovine serum albumin (BSA) marker. Fig.(1). High and low molwcular weight as protein marker were sued to estimate several major and minor bands Table (2)
The results revealed that the molecular weight of protein samples were around 66 kDa. Six protein bands were identefied ranged from 16.6 to 75kDa in molecular weight, Fig(2) and Table (3). A single protein pattern with a molecular weight of 16.6 kDa was observed for all insect stages. The lab strain adults revealed two protein patterns with molecular weight of 19.1 and 52 while the field strain adult produced a total protein of 52 and 16.6 kDa. Four protein bands with molecular weight of 16.6, 58.8, 69 and 75.6 kDa were identified for pupa (Table 3). This data consistent with other data reported by Lee et al.,2009 who found similar protein bands, which fall in the range of approximately 200 kDa to less than 24 kDa. This result in agreement with Rohani, et al ., 2005 who mentioned that the protein bands of Ae.aegypti were within the range of 14 – 80 kDa. Study of Junsuo and Jianyong 2006 described total and specific protein isolated from matures and immature stages of Ae.aegypti, Another researcher revealed that the isolated haemolymph proteins profiles of adult female Aedes togoi varied from 10-80 kDa (Jariyapan et al., 2011) Proteins with molecular weights of 240 and 70 kDa were reported ( Capurro et al., 1994; Ford and Van Heusden, 1994; Van Heusden et al., 1998).
.Several studies about mosquitoes protein have been reported in different parts in the world (Biessmann et al., 2002 , Sun et al., 2000. Von-Dungern and Briegel, 2000, 2001 Pimsamarn , et al., 2009 , Jariyapan et al., 2007 and Valenzuela et al., 2002).
As showed in Fig.(1) several major and minor bands were appeared with high resolution comparing with bands in Fig(2) which appeared to be slightly poor in resolution (fainted or invisible band) and number of bands, this might be due to volume of extracted protein sample (15 µl) that was loaded as compared to 25 µl in Fig(2) Moreover the electrophoresis conditions in Fig (1) such as the concentration of Bromophenol blue dye, stained and the running time which was 2hrs in Fig(1) compared to 6 hrs. in Fig(2)
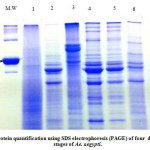 |
Figure 1: Protein quantification using SDS electrophoresis (PAGE) of four development stages of Ae. aegypti.
|
Where:
M Marker
1 = larvae,
2 = Lab adult,
3 = Pupae,
4 and 5 = Field adult,
6 = Pupae
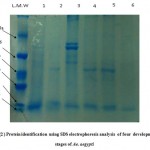 |
Figure 2: Protein identification using SDS electrophoresis analysis of four development stages of Ae. aegypti.
|
Where:
L M W Low molecular weight kDa,
H M W High molecular weight kDa,
1 = larvae
2 = Lab adult,
3 = Pupae,
4 and 5 = Field adult
,6 = Pupae
Table 2: Molecular weight of protein marker Used in electrophoretic SDS –PAGE
| Markers | Molecular weight KDa |
| Phosphorylase | 97 |
| Albumin | 66 |
| Ovalbumin | 45.709 |
| Carbonicanydrass | 30 |
| Trypsin inhibitor | 20 |
| Lactabulmin | 14.4 |
Log molecular weight
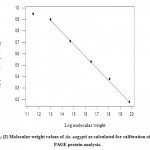 |
Figure 3: Molecular weight values of Ae. aegypti as calculated for calibration of SDS-PAGE protein analysis.
|
The calibration curve was constructed by plotting the electrophoresis mobility of standard proteins versus the logarithms of their corresponding molecular weight values. Unknown molecular weight of total protein of Ae. agyptian development stages were determined by measuring the electrophoretic mobility of the unknown total protein and the corresponding molecular weight from the curve as showed in Fig.(3) and table (3)
Table 3; Total protein of different developmental stages (larvae, pupae and adults) using SDS-PAGE electrophoresis
| Lane No. | Band No. | Insect stage | Molecular weight KDa |
| 1 | 1 | Larva | 16.6 |
| 2 | 2 | Adult (Lab) | 51.7 |
| 3 | 19.1 | ||
|
3 |
4 |
Pupa |
75.6 |
| 5 | 69 | ||
| 6 | 58.8 | ||
| 7 | 16.6 | ||
|
4 |
8 |
Adult (Field)
|
52 |
| 9 | 16.6 | ||
| 5 | 10 | 52 | |
| 11 | 16.6 | ||
| 6 | 12 | 16.6 |
Conclusion
This study described and identified ten distinct of Ae. aegypti developmental stages as a total protein . These results provide basic information and an initial step for identification of differentially specific proteins of Ae. aegypti.. Further studies are needed for identifying specific proteins and their function and biological roles in dengue fever infections. Proteomics study is necessary for understanding developing novel vector control strategies and parasite-vector interactions, gene expression which will be dominate post- research.
Acknowledgments
We gratefully acknowledge the king Abdulaziz city for science and technology( kacst) for their financial support under grand No.( A-S-10-0013) .We would also like to express our deep appreciation to Prof. Dr Mahmoud Abo-El – Saad for his helping in practical work. I deeply thank Dr. Magdi Mosa for valuable guidance and reviews of part of draft as well as Dr. Ahmed Bakhashwain for generous support of requirements chemicals in biotechnology lab.
References
- Abo-El-Saad, M. and A. Ajllan. (2003). Zymography and radial diffusion analysis of trypsin-like enzyme from midgut of red palm weevil Rhynchophorus ferrugineus (Olivier). Alex. J. Pharma. Sci. 17, 95-99
- Bakr, Helmy N, Nawwar G, El. IbrahimS and Helmy O (2010)Changes in protein content of Culex pipiens mosquito treated with two agriculture waste extracts. Acad. J. biolog. Sci., 3 (1): 95- 103
- Berlocher ,S.H. (1984) Insect molecular systematics. Ann. Rev. Entomol. 29: 403-433.
- Biessmann H, Walter M, Dimitratos S and Woods D (2002) Isolation of cDNA clones encoding putative odourant binding proteins from the antennae of the malaria transmitting mosquito, Anopheles gambiae. Insect Mol.. Bio.11,(2) 123-132
- Chee H.-Y. and . AbuBakar S (2004) Identification of a 48 kDa tubulin or tubulin-like C6/36 mosquito cells protein that binds dengue virus 2 using mass spectrometry. Biochemical and Biophysical Research Communications 320.
- Crawford, D. and Ornduff, R(1989) Enzyme electrophoresis and evolutionary relationships among three species of Lasthenia (Asteraceae: Heliantheae). Am. J Bot., 76(2): 289-296.
- Capurro, M.de L., de Bianchi, A.G and Marinotti, O.(1994). Aedes aegypti lipophorin. Comp. Biochem. Physiol. 108, 35–39.
- Darsie , R . and Ward , R ( 2005 ) Identification and geographical distribution of the mosquitoes of North America, North of Mexico. J. Amer. Mosq. Cont. Assoc. ,21: 1– 383.
- Ford, P. and Van Heusden, M (1994) Triglyceride-rich lipophorin in Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 31, 435–441.
- Ishida Y , .Cornel A and Leal W (2002) Identification and cloning of a female antenna-specific odorant-binding protein in the mosquito Culex quinquefasciatus. J. Chem. Eco. 28 -867-871
- Gupta S and Preet S(2012) Protocol optimization for genomic DNA extraction and RAPD-PCR in mosquito larvae (Diptera: Culicidae) Annals of Biological Research. 3 (3):1553-1561
- Hung, J, Hsieh, M., Young.(2004). An external loop region of domain III of dengue virus type 2 envelope protein is involved in serotype-specific binding to mosquito but not mammalian cells. J. Viro.78 (1):378-388.
- Jariyapan N, Choochote W, Jitpakdi A, Harnnoi T, Siriyasatein P, Wilkinson M, Junkum A and Bates P (2007) Salivary gland proteins of the human malaria vector, anopheles dirus b (Diptera: Culicidae). Rev. Inst. Med. trop. S. Paulo 49(1):5-10
- Jariyapan .N Uparanukraw P, Wannasarn A, Saeung A, Khositharattanakoo P, Sor-suwan S and Phattanawiboon B (2011)Analysis of haemolymph proteins in the brugia malayi-susceptible mosquito, Aedes togoi, using SDS-PAGE and two-dimensional gel electrophoresis. International J. Parasitol. Res.. 3:(2) 31-38
- Junsuo S. L and Jianyong L(2006)Major chorion proteins and their crosslinking during chorion hardening in Aedes aegypti mosquitoes. Insect Biochem Mol Biol.36(12): 954–964
- Laemmli, U. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227-280.
- Lee , H., Wong, Y. and Rohani, A. (2009) Protein profiles of dengue-infected Aedes aegypti (L).Dengue Bulletin – Volume (33)115-123
- Nevo, E., Ben-Shlomo, R. and Lavie, B. (1984) Mercury selection of allozymes in marine organisms: prediction and verification in nature. Proceedings of the National Academy of Science USA 81: 1258–1259.
- Onarici G. and sumer S.(2003). Protein and DNA in Systematic Biology. Turk J Biol.(27):47-55.
- Pimsamarn S , Sornpeng W, Akksilp S and. Limpawitthayakul M (2009) Detection of insecticide resistance in Aedes aegypti to organophosphate and synthetic pyrethroid compounds in the north-east of Thailand. Dengue Bulletin – ( 33)194-202
- Popova-Butler A and Dean D (2008) Protemic analysis of the mosquito Aedes aegypti midgut brush border membrane vesicles. J Insect Physol.55(3):264-272
- Rohani, A., Yunus, W., Zamree, I. and Lee, H.. (2005) Protein synthesized by dengue infected Aedes aegypti and Aedes albopictus. Tropical Biomedicine 22(2): 233–242 .
- Sheppard W S. and Smith D. R(2000). Identification of African-Derived Bees in the Americas: A Survey of Methods. Annals of the Entomological Society of America: (93) 159-176
- Smith I. (1976).Chromatographic and electrophoretic techniques. Vol. 2. London London:Heineman.485p
- Studier, F. (1973) Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol. Biol. ;79:237–248.
- Sun J, Hiraoka T, Dittmer N, Cho K and Raikhel A (2000) Lipophorin as a yolk protein precursor in the mosquito, Aedes aegypti. Insect Biochem Mol. Bio. 30 :1161–1171.
- Valenzuela J.G , Pham V.M, Garfield M.K, Francischetti I.M and Ribeiro J.M (2002)Toward a description of the sialome of the adult female mosquito Aedes aegypti. Insect Biochem. and Mol.Biol. (32 )1101–1122
- Van Heusden, M.C., Thompson, F., Dennis, J., (1998). Biosynthesis of Aedes aegypti lipophorin and gene expression of its apolipoproteins. Insect Biochem. Mol. Biol. 28, 733–738
- Von Dungern, P. and Briegel, H.( 2000). Enzymatic analysis of uricotelic protein catabolism in the mosquito Aedes aegypti. J. Insect Physio. 47, 73–82.
- Von Dungern, P and Briegel, H (2001) Protein catabolism in mosquitoes: ureotely and uricotely in larval and imaginal Aedes aegypti. J.Insect Physio. 47 :131–141
- Whitehorn J and Farrar J (2010). “Dengue”. Br. Med. Bull. 95: 161–73
- WHO (2003). Guidelines for Surveillance and Mosquito Control. Sec. ed. WHO Regional Office Edu. In Action Series 8-12.
- WHO (2000). Dengue/dengue haemorrhagic fever. Weekly Epidemiol. Re.75:193-196.
- Wood, D.., Dang, P. and Ellis, R.. ( 1979 ) The mosquitoes of Canada. Diptera: Culicidae . In : The Insects and Arachnids of Canada ,Part 6. Agri. Canada Public. , 1–390.

This work is licensed under a Creative Commons Attribution 4.0 International License.





