How to Cite | Publication History | PlumX Article Matrix
Anar Nurgaiypovna Kaliyeva, Gulzhamal Unalbaevna Dyuskaliyeva, Rysmakhambet Konirovish Zheхembiyev, Galiya Dzhumahanovna Medeuova and Zhanar Slyamkhanovna Tileubayeva
Kazakh State Women’s Teacher Training University, Kazakhstan, 050000, Almaty, Aiteke bi Street, 99
ABSTRACT: The paper presents morphological and anatomical data on medicinal plant Agrimonia L. growing in the south-east of Kazakhstan. Here you can find the data on peroxidase activity of leaves, stems and roots, as well as analysis of total proteins concentrations in the stems of different populations of medicinal plants Agrimonia asiatica Juz., Agrimonia eupatoria L., Agrimonia pilosa Ldb. The spectrum of cathode isoforms of all organs of plants Agrimonia pilosa Ldb. from the 4th population shows specificity by activity and heterogeneity of the spectrum.
KEYWORDS: Agrimonia asiatica Juz; Agrimonia pilosa Ldb; Agrimonia eupatoria L.; population; phloem; xylem; epidermis; isoform; peroxidase; esterase; cytoplasmic proteins
Download this article as:| Copy the following to cite this article: Kaliyeva A. N, Dyuskaliyeva G. U, Zheхembiyev R. K, Medeuova G. D, Tileubayeva Z. S. Anatomical, Morphological and Biochemical Analysis of Medicinal Species Agrimonia L. Growing at South-East of Kazakhstan. Biosci Biotech Res Asia 2015;12(2) |
Introduction
The role of Medicinal Plants
Conservation and sustainable use of plant resources of our planet is now a global issue of interstate level (Proskuriakov M.A., 2012). Many years of experience in the study of medicinal plants shows that their extractions have low toxicity and possess the necessary medicinal properties. The variety of biologically active substances provides a wide range of pharmacological effects of herbal medicines (Goldberg E.D. and Zueva E.P., 2000). The flora of Kazakhstan is an inexhaustible source of vegetable raw materials in particular in the mountain areas. Medicinal plants serve as a valuable raw material for a wide range of herbal remedies and therapeutic pharmacological actions that are fast-acting, do not have cumulative effect and cause adverse effects to a lesser extent. Medicinal plants of Kazakhstan contain most of the known classes of biologically active substances. According to the phytochemical composition medicinal plants possess a very broad spectrum of pharmacological actions (Grudzinskaya L.M. et al., 2014). Plant Agrimonia L. of the wild flora of Kazakhstan is an issue of the particular interest for its pharmacologically promising species. Due to this fact the study of the direction of change in genetic and biochemical traits in populations of valuable species of plants growing in different environmental conditions has a great theoretical and practical interest.
Therapeutic properties and medical use
Biochemistry of plants represents rich and diverse source materials and tools that provide healthy, happy and full-fledged lives of people. A.pilosa Ldb. may be a good source of natural antioxidants and alpha-glucosidaseinhibitors exhibiting remarkable potential value for the therapy of T2DM (By Liu. et al., 2014). Nine compounds were isolated from the active section with lowering blood sugar of agrimony, and their structures were identified as oleanoic acid, ursolic acid, 19-alpha-hydroxy ursolic acid, tormentic acid, apigenin, luteolin, kaempferol, 3,3′-di-O-methyl ellagic acid, and kaempferol-7-O-alpha-L-rhamnoside (Cehn YS. et al., 2010). Few previous reports have investigated the chemical components of A.pilosa Ldb. Chemical studies on A.pilosa L. have shown the presence of polyphenols such as flavonoids (Kato H. et al., 2010). In a bioassay-guided search for acetylcholinesterase (AChE) inhibitors from 180 medicinal plants, an ethyl acetate extract of whole plants of Agrimonia pilosa ledeb yielded tiliroside, 3-methoxy quercetin, quercitrin and quercetin. We report herein for the first time that all four flavonol compounds showed significant inhibitory effects on AChE, particularly quercetin. This showed the activity of dehydroevodiamine (DHED) (Jung M. and Park M., 2007). A.pilosa Ldb. has potent estrogenic activity and may have beneficial effects for postmenopausal women requiring ERT (Lee YM. et al., 2012). A. pilosa Ldb. extract shows an antinociceptive property in various pain models. Furthermore, this antinociceptive effect of A. pilosa Ldb. extract may be mediated by α2- adrenergic receptor, but not opioidergic and serotonergic receptors (Soo-Hyun Park. et al., 2012). Essential oils, tannins, vitamin K, flavonoids (quercetin and others), choline, bitterness, silicic acid, catechins, triterpenes, and organic acids were found in A.eupatoria L. Medical drugs based on sarcocolla were used as anti-inflammatory, antispasmodic, expectorant, diaphoretic, choleretic and diuretic agents.
The purpose of the current study is to reveal morphological and anatomical structure of vegetative organs, activity of proteins, esterase and peroxidase in the aboveground and underground parts of plants of the genus Agrimonia L. collected in different populations.
Methods
Objects of the study were the following: Agrimonia asiatica Juz. – asian agrimony, Agrimonia pilosa Ldb.- hairy agrimony, Agrimonia eupatoria L. – ordinary agrimony from the family Rosaceae Juss growing at the southeast of Kazakhstan.
Materials and methods of anatomical and morphological study
The materials for the study were samples of plants of A.asiatica Juz. (1-2) population growing in the village of Koldi of the Main Botanical Garden of Almaty. A.eupatoria L. was collected at the territory of the Main Botanical Garden of Almaty. Whereas samples of the plant A.pilosa Ldb. were collected from the populations growing at the mountain areas at the territory of the National Park (NP) called Kolsai lakes. Specific definition of the studied plants was carried out according to the Flora of Kazakhstan and illustrated determinant of the plants of Kazakhstan (Kazakhstan Flora.1961, Illustrated determinant of the plants of Kazakhstan, 1969). Based on the material collected in 2012-2013 and preserved in the ratio of alcohol, glycerol, and water as 1:1:1 respectively for Strasburger-Flemming, anatomical and morphological studies were conducted according to the procedure of M.N. Prozina (Prozina M.N., 1960). Anatomical sections of plants were made using a microtome MZP-01 “technom”, Ekaterinburg. For the quantitative analysis we measured the morphometric parameters using a microscope MA 100 micros Austria equipped with photo attachment and lenses x4/0.10, magnification EW 10x/20.
Materials and methods of biochemical study
Objects of the research for biochemical analysis were 3 types of plants of the genus Agrimonia L. Samples of plants A.asiatica Juz. were collected from four different populations (1-4) growing at the territory of the village of Koldi at the Main Botanical Garden of Almaty and near to the hole Butakovka on the territory of health-improving and recreation complex Akbulak. Samples of plants A.eupatoria L. were collected at the territory of the Main Botanical Garden of Almaty. Whereas samples of plants of the species A.pilosa Ldb. were collected from the mountain populations (1-4) at the territory of the village of Sata, Tomarsaz; Karasai; National Park – NP Kolsay.
Method for the determination of peroxidase activity
Peroxidase isolated from leaves, stems and roots by grinding in liquid nitrogen and exposure to tris-glycine buffer with pH 8.3. Total peroxidase activity was measured by the photo-calorimetry FEC 56 by the velocity of oxidation of benzidine by Boyarkin (Boyarkin A.N., 1951). Translation of the activity into nanokatals was conducted by Lebedeva O.V. (Lebedeva O.V. et al., 1977). Electrophoretic separation of cathodic peroxidases was performed in tris-glycine buffer at pH 8.3 (Kabzhanova S.B. and Peruasky Y.V., 1975). We used Thermoscientific kits (Lithuania) as a molecular weight marker comprising a mixture of purified protein with a molecular mass from 10 to 200 kDa.
Methods for detection of cytoplasmic protein
Extraction of cytoplasmic proteins was performed in phosphate buffer pH 7.5. An extract was centrifuged at 10 000 rpm. A chilled acetone was added to the supernatant. The precipitate was re-dissolved in 0.0618 M tris-HCl buffer by the solution containing SDS Na – 3%, glycerol 10%, merkaptetonol 4% and bromophenol blue dye. The sample was alkylated, heated for two minutes in a boiling water bath. 24 µl of the sample was added into the pockets of 10% polyacrylamide gel. Preparation of the gel and electrophoresis was carried out using the Laemmli (1970) method modified by Bulatova K.M. (Bulatova K.M., 1985). Thermoscientific kit, Lithuania was used as molecular weight marker (200 kDa, 150 kDa, 120 kDa, 100 kDa, 85 kDa, 70 kDa, 60 kDa, 50 kDa, 40 kDa, 30 kDa, 25 kDa, 15 kDa, 10 kDa).
Method of determining the peroxidase activity
Tissue extract from various types of Agrimonia L. was used for the analysis of peroxidase enzyme. It was also used for fractionation of esterases. In order to display isoforms esterase, we used alpha- and beta-naphtylacetate as a substrate. This substrate is the most preferred one for the imaging of nonspecific esterase (Peskan T.et al., 1996).
Results and Discussion
Botanical characteristics of the genus Agrimonia L.
Asian agrimony (A.asiatica Juz. belongs to the family Rosaceae Juss. It is perennial, rather tall plant (up to 80-117 cm) with a straight stem and long branches. Number of stems per plant is 10-14. Rhizome is branching with a length of 15-20 cm. Leaves are green on the top, densely adjacent and piliferous, gray and green on the bottom, densely and velvety-hairy, with small yellow glands. Plant has 70-157 leaflets, with a length of 2-8 cm, and a width of 1-4 cm, elliptic, oblong-ovate, large and sharp-toothed. Inflorescence is a spicate acervulus with straddling at the bottom, and closely spaced flowers and fruits at the top. Flowers have short pedicels, 5 calyx lobes and 5 golden yellow petals. Fruits have spines (Kalieva A.N. and Dyuskalieva GU, 2014).
 |
Figure 1: Morphology of medicinal species of the genus Agrimonia L.: A-stem, B – fruit, C – leaves, D-root |
Hairy agrimony (Agrimonia pilosa Ldb.) is a perennial plant that belongs to the family Rosaceae Juss. It reaches 63 to 90 cm in height. The stem is cylindrical, branched, covered with hairs. Leaves are straddling, broken-pinnate, naked or sparsely hairy, green on both sides, darker on top. The number of leaves on one plant was 96 ± 2.01. Inflorescence is a spicate acervulus, 6-8 mm in diameter. Fruits are erect and droop only after full maturation. Petals are oblong, pale yellow, branching rhizome (Kalieva A.N. and Dyuskalieva G.U., 2014).
Ordinary agrimony (Agrimónia eupatória L.) is a perennial plant from the rose family. Plants have 60-150 cm in height, straight stem covered by greenish-gray, fluffy hair as well as leaves. Leaves are intermittent pinnate, elliptic, toothed. Flowers have five yellow petals, 6-12 mm in sizes, inflorescence is a spicate acervulus. Flowering is in June – August. The fruit is a single coccus enclosed in a dry hypanthium with hooked spines.
Anatomical features of medicinal species of the genus Agrimonia L.
Anatomical structure of A.asiatica Juz. vegetative organs.
In the internal structure of the limb there are well-developed simple trichomes on the lower epidermis. On the main vein there is a large conductive fascicle with well-developed xylem vessels. On cross-section of the stem A.asiatica Zus. xylem vessels have developed twice more than phloem structures and friable parenchyma cells with the round shape. On the cross-section of root there are xylem fascicles of different sizes.
Anatomical structure of A.eupatoria L. vegetative organs.
In cross-section of the leaf on both sides of epidermis there are trichomes, columnar and spongy mesophyll in a single row. In the center of a leaf there is collanterial, larger conductive fascicle with well-developed xylem vessels. On cross-section of the stem there is a single row epidermis, consisting of small cells and vascular bundles. The core consists of a friable, rounded parenchyma cells. Root on the cross section has a rounded shaped. Its internal regions occupy the central cylinder. Phloem is located between the xylem fascicles. Xylem is composed of vessels of different sizes.
Anatomical structure of A. pilosa Ldb. vegetative organs.
Cross section of the leaf reveals epidermal cells, columnar and spongy mesophyll from the external surface. Leaf has a dorsoventral structure. On the main vein there are two collateral, closed-type vascular bundles. Xylem elements are well developed. Simple and edgeless cone-shaped trichomes are located on both sides of the leaf. Collateral vascular bundles of the stem have a close localization on the surface of the stem, evenly in a single row. In the stem xylem vessels evolved twice more than the elements of the phloem. By the way there was bass lining of the fascicles. On the cross section of the root xylem, fascicles were more developed than the phloem.
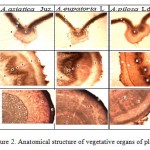 |
Figure 2: Anatomical structure of vegetative organs of plants: |
A – leaves: 1 – upper epidermis, 2 – lower epidermis, 3- spongy mesophyll, 4-columnar mesophyll, 5- xylem, 6- phloem, 7- conductive fascicle; 8 – trichomes; B – stem: 1 – epidermis, 2 – primary bark, 3 – sclerenchyma, 4 – phloem, 5 – xylem, 6 – parenchyma core, 7- conductive fascicle, 8 – trichomes; C – root: 1 – periderm, 2-primary bark, 3 – secondary bark, 4 – phloem, 5 – cambium, 6 – xylem.
Biochemical Analysis of the Species of the Genus Agrimonia L.
Electrophoretic analysis of proteins and isozymes remains to be one of the easiest and most affordable biochemical methods allowing clear identification of not only individual species but also subspecies, populations or biotypes of plants for the minimal cost (Peterson A.N. et al., 1991; Khavkin E.E., 1997). Enzymes are substances of protein nature which are able to accelerate chemical reactions in the body and serve as organic catalysts. They also play an important role in metabolism. Peroxidase enzyme belongs to the class of oxidoreductases. By its chemical composition it is a heme-containing glycoprotein that catalyzes the oxidation of several substrates by hydrogen peroxide (Baranova T.V. et al., 2001). Active groups of peroxidase contain iron, hydrogen peroxide. It is activated and acts as a hydrogen acceptor. Hydrolases catalyze the hydrolysis and sometimes synthesis of organic compounds with water bonding to it. The most important are esterases which accelerate the hydrolysis reaction and synthesis of esters. Oxidoreductases are enzymes that accelerate the oxidation-reduction reactions. These include oxidase, peroxidase and catalase involved in the breathing process. Hydrolases are enzymes which catalyze cleavage of complex organic compounds with participation of water. Esterase catalyzes the cleavage and synthesis of esters.
Total peroxidase activity of medicinal species of the genus Agrimonia L.
Total peroxidase activity of leaves in Agrimonia L. species varies from 311 to 1,290 of nanokatales. It should be noted that total peroxidase activity is the highest in A.pilosa Ldb. from population 4. Total enzyme activity of stems in the species A.asiatica Juz. and A.eupatoria L. is higher than that in the stem of A.pilosa Ldb. Peroxidase activity in roots is the highest in species A.pilosa Ldb. (Table 1).
Table 1: Total peroxidase activity of leaves, stems and roots of species Agrimonia L. (A. asiatica Juz., A.eupatoria L., A.pilosa Ldb.)
| Species, population | ||||||||||
| E1 | Nanokatales | E2 | Nanokatales | E3 | Nanokatales | Emean | Nanokatales | |||
| Leaf | ||||||||||
| A.asiatica Juz, p.1 | 0.109 | 484 | 0.111 | 493 | 118 | 524 | 0.113 | 502 | ||
| A.asiatica Juz., p.2 | 0.107 | 475 | 0.109 | 484 | 0.104 | 462 | 0.106 | 471 | ||
| A.asiatica Juz. p.3 | 0.122 | 542 | 0.118 | 524 | 0.125 | 555 | 0.121 | 537 | ||
| A.asiatica Juz. p.4 | 0.072 | 320 | 0.071 | 315 | 0.068 | 302 | 0.070 | 311 | ||
| A.eupatoria L. | 0.085 | 377 | 0.090 | 400 | 0.092 | 408 | 0.089 | 395 | ||
| A.pilosa Ldb. p.1 | 0.122 | 542 | 0.132 | 586 | 0.127 | 564 | 0.127 | 564 | ||
| A.pilosa Ldb. p.2 | 0.071 | 315 | 0.086 | 382 | 0.075 | 333 | 0.077 | 343 | ||
| A.pilosa Ldb. p.3 | 0.105 | 466 | 0.112 | 497 | 0.108 | 481 | 0.108 | 481 | ||
| A.pilosa Ldb. p.4 | 0.295 | 1311 | 0.286 | 1271 | 0.290 | 1290 | 0.290 | 1290 | ||
| Stem | ||||||||||
| A.asiatica Juz, p.1 | 0.242 | 1075 | 0.250 | 1111 | 0.244 | 1084 | 0.245 | 1089 | ||
| A.asiatica Juz., p.2 | 0.158 | 702 | 0.154 | 684 | 0.155 | 689 | 0.156 | 693 | ||
| A.asiatica Juz. p.3 | 0.251 | 1115 | 0.268 | 1191 | 0.262 | 1164 | 0.260 | 1155 | ||
| A.asiatica Juz. p.4 | 0.490 | 2178 | 0.480 | 2133 | 0.482 | 2142 | 0.484 | 2151 | ||
| A.eupatoria L | 0.375 | 1667 | 0.388 | 1724 | 0.377 | 1675 | 0.380 | 1689 | ||
| A.pilosa Ldb. p.1 | 0.120 | 533 | 0.109 | 484 | 0.117 | 520 | 0.115 | 511 | ||
| A. pilosa Ldb. p.2 | 0.102 | 453 | 0.105 | 466 | 0.104 | 462 | 0.103 | 458 | ||
| A.pilosa Ldb. p.3 | 0.115 | 511 | 0.118 | 524 | 0.119 | 529 | 0.117 | 520 | ||
| A. pilosa Ldb. p.4 | 0.152 | 675 | 0.155 | 689 | 0.152 | 675 | 0.153 | 680 | ||
| Root | ||||||||||
| A. asiatica Juz, p.1 | 0.105 | 466 | 0.101 | 449 | 0.102 | 453 | 0.103 | 457 | ||
| A. asiatica Juz., p.2 | 0.110 | 488 | 0.101 | 449 | 0.107 | 475 | 0.106 | 471 | ||
| A. asiatica Juz. p.3 | 0.109 | 484 | 0.106 | 472 | 0.105 | 466 | 0.107 | 475 | ||
| A. asiatica Juz p.4 | 0.118 | 524 | 0.107 | 475 | 0.112 | 497 | 0.117 | 520 | ||
| A.eupatoria L. | 0.121 | 537 | 0.119 | 529 | 0.118 | 524 | 0.119 | 528 | ||
| A. pilosa Ldb. p.1 | 0.115 | 511 | 0.113 | 502 | 0.111 | 493 | 0.114 | 506 | ||
| A.pilosa Ldb. p.2 | 0.111 | 493 | 0.112 | 497 | 0.111 | 493 | 0.111 | 493 | ||
| A.pilosa Ldb. p.3 | 0.119 | 529 | 0.115 | 512 | 0.122 | 542 | 0.118 | 524 | ||
| A.pilosa Ldb. p.4 | 0.158 | 702 | 0.167 | 742 | 0.162 | 720 | 0.162 | 720 | ||
Results of Peroxidases electrophoresis conducted under alkaline conditions
Peroxidases electrophoresis is conducted under alkaline conditions. This procedure showed virtually no signs of the activity of all isoforms of the cathode in species A.asiatica Juz. and A.eupatoria L. After fractionation of peroxidases of the species A.pilosa Ldb. it was noted that the highest activity is attributed to the isoforms with molecular weight of 35-70 kDa. Moreover, the most active isoforms are those with a molecular weight of 37-40 kDa. The spectrum of cathode isoforms of all organs of plants of the 4th population of this species shows specificity both in activity and heterogeneity of the spectrum (Figure 3).
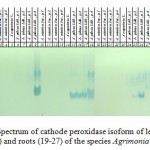 |
Figure 3: Spectrum of cathode peroxidase isoform of leaves (1-9), stems (10 -18) and roots (19-27) of the species Agrimonia L., 28-marker |
An absence of activity signs in the spectrum of peroxidase isoforms of the species A.asiatica Juz. and A.eupatoria L. is apparently caused by their inhibition by phenolic compounds. Basic compound of water-alcoholic extract of A.eupatoria L. are flavonoids. Extract also contains large amounts of tanines and has a high content of phenolic compounds which have an inhibitory effect on the enzymes. Antioxidant activity of A. eupatoria L. might be due to chemical structure of polyphenols and herb ability to activate the endogenous antioxidant defence systems (Ivanova D. et al., 2011). Polyphenol profile of Agrimonia herbs, antioxidant activity and inhibition of α-glucosidase support the traditional use of these plants as anti-diabetic and anti-inflammatory drug (Kubinova R. et al., 2012). Enzymes that are capable to neutralize superoxide radicals and peroxyl species in the cells play the main role in the protection from reactive oxygen intermediates. Superoxide dismutase (SOD) destroys superoxide anion radicals to hydrogen peroxide. Catalase reduces hydrogen peroxide to water and oxygen. Peroxidase reduces hydrogen peroxide to water but with participation of organic reducing agents (Menytsikova E.B. and Zenkov N.K., 1993).
The results of electrophoresis of cytoplasmic proteins of the stems of species Agrimonia L.
Proteins in all organisms are produced on the matrices – molecules of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). These molecules are able to preserve and carry hereditary characteristics of organisms through thousands of generations. All other products formed by proteins serve as a base to form proteins, which are identical or very similar in all living beings.
Electrophoresis of cytoplasmic proteins isolated from the stems of plants Agrimonia asiatica Juz., Agrimonia eupatoria L., Agrimonia pilosa Ldb. presented by different populations did not reveal any diversity between species and between populations (Figure 4).
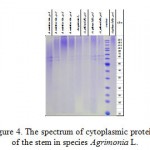 |
Figure 4: The spectrum of cytoplasmic proteins of the stem in species Agrimonia L. |
Proteins were presented by two intense and several minor bands. They were expressed most clearly in the electrophoretic spectrum of the molecular weight from 60 to 70 kDa.
Character of manifestation of esterase isoforms in the species of Agrimonia L.
In order to display isoforms, we performed electrophoresis in 10% polyacrylamide gel. Then in a 100 mL glass plate we poured 20 ml of tris-HCl buffer and 80 ml of distilled water. 5 minutes before staining the gel 50 mg naphtylacetate was dissolved in 5 ml of acetone and then was slowly poured in the buffer with stirring. The gel was transferred to this solution. Pre-filtered and dissolved BB salt (50 mg in 10 ml distilled water) was added to gel-containing cuvette (Figure 5).
 |
Figure 5: Character of manifestation of esterase isoforms after 15min, 30 min and 50 min exposure in the solution. |
First bands were thin and weak. After coloring they became wider and more intense. In the spectrum of esterase in species Agrimonia L. there are 2 groups of isoforms. Isoforms are present most intensively in the spectrum related to stems and leaves of A.pilosa Ldb. (Figure 6).
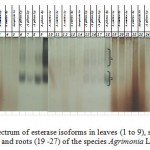 |
Figure 6: Spectrum of esterase isoforms in leaves (1 to 9), stems (10 – 18) and roots (19 -27) of the species Agrimonia L. |
It should be noted that the activity of isoforms in populations selected in NP Kolsay – (A.pilosa Ldb., 4p.) was the highest in both cases.
Conclusion
The study revealed particular features of anatomical and morphological structure of medicinal species of the genus Agrimonia L. growing in the south-eastern region of Kazakhstan. Xylem vessels can be clearly visualized in the cross-section of the stem of A.asiatica Juz., A.eupatoria L. In the lower epidermis of leaf there are simple trichomes. Xylem fascicles can be clearly visualized in the cross-section of the root. Vascular bundles of the stem of A.pilosa Ldb. are collateral, xylem vessels are more expressed in the stem. There was developed bast-fiber sheath. Analysis of the data showed that there are no apparent differences in the anatomy of the species A.asiatica Juz., A.eupatoria L. According to its therapeutic action, A.pilosa Ldb. is similar to A.asiatica Juz. It differs from it only in small morphological features. In addition to the main vein there are two vascular bundles of the collateral type. Total peroxidase activity in the leaves and roots of A.pilosa Ldb. from the 4th population is the highest. Total enzyme activity of stems is higher in A. asiatica Juz. and A.eupatoria L. species than in A.pilosa Ldb. species. The result of peroxidases electrophoresis conducted under alkaline conditions revealed no signs of the activity of the cathode isoforms of all organs in A. asiatica Juz. and A.eupatoria L. species. All plant organs in A.pilosa Ldb. species of the population 4 are active. Esterase activity of the stem and leaf is high in A.pilosa Ldb. species, 4th population. In roots an isoform activity is manifested in trace amounts.
Acknowledgments
We express our special gratitude to Bulatova K.M., Doctor of Biological Sciences, Head of the Laboratory of Molecular-biological Analysis of Plants at the Kazakh Research Institute of Agriculture and Plant, LLC, for her valuable advice and assistance in carrying out the research.
References
- Proskuriakov, M.A. 2012. Chronobiological analysis of plants with changes of the climate. Almaty: Pubslishing House of LEM, pp: 230.
- Goldberg, E.D. and E.P. Zueva, 2000. Drugs from plants in the complex therapy of malignant diseases. Tomsk: Pubslishing House of Tomsk University, pp: 125.
- Grudzinskaya, L.M., N.G. Gemedzhieva, N.V. Nelina and J.J. Karzhaubekova, 2014. Annotated list of medicinal plants in Kazakhstan. Almaty: Pubslishing House of Almaty, pp: 4-10.
- Boyarkin, A.N., 1951. Quick method for determining peroxidase activity. Biochemistry, Vol. 16, 4: 352-355.
- Lebedeva, O.V., N.N. Ugarova and I.V. Berezin, 1977. Kinetic study of the oxidation reaction of ortho-dianisidine by hydrogen peroxide in the presence of HRP. Biochemistry, Vol. 42, 8: 1372-1379.
- Kabzhanova, S.B. and Y.V. Peruvian, 1975. Composition and activity of peroxidase isozymes and esterases of heterosis maize hybrids. Bulletin of Agricultural Science of Kazakhstan, 9: 33-37.
- Bulatova, K.M., 1985. The study of the component composition of wheat glutenin. Bulletin of Agricultural Science of Kazakhstan, 4: 37-39.
- Liu, X., L.C. Zhu, J. Tan, X.M. Zhou, L. Xiao, X. Yang and B.C. Wang, 2014. Glucosidase inhibitory activity and antioxidant activity of flavonoid compound and triterpenoid compound from Agrimonia pilosa Ledeb. BMC Complementary and alternative medicine. Journal Information, 14:12. DOI: 10.1186/1472-6882-14-12.
- Cehn, Y.S., K. Zhang, S.Q. Zhao and J.H. Zhang, 2010. Studies on the lowering blood sugar substances from agrimony (II). Zhong Yao Cai, 33(5): 724-6. PMID: 20873555 [PubMed – indexed for MEDLINE].
- Kato, H., W. Li, M. Koike, Y. Wang and K. Koike, 2010. Phenolic glycosides from Agrimonia pilosa. Phytochemistry, 71(16): 1925-1929. doi: 10.1016/j.phytochem.2010.08.007. [PubMed] [Cross Ref].
- Jung, M. and M. Park, 2007. Acetylcholinesterase inhibition by flavonoids from Agrimonia pilosa. Molecules, 12(9): 2130-2139. doi: 10.3390/12092130. [PubMed] [Cross Ref].
- Lee, Y.M., J.B. Kim, J.H. Bae, J.S. Lee, P.S. Kim, H.H. Jang and H.R. Kim, 2012. Estrogen-like activity of aqueous extract from Agrimonia pilosa Ledeb. in MCF-7 cells. National Academy of Agricultural Science, Rural Development Administration, Suwon, Republic of Korea. BMC Complement Altern Med., 12: 260. doi: 10.1186/1472-6882-12-260.
- Park, S.H., Y.B.Sim, Y.J.Kang, J.K. Lee, S.S.Lim and H.W.Suh, 2012. Effect of Agrimonia pilosa Ledeb Extract on the Antinociception and Mechanisms in Mouse. Korean Journal Physiology Pharmacology, 16: 119-123. http://dx.doi.org/10.4196/kjpp.2012.16.2.119.
- Peterson, A.N., S.D. Tanksley and M.E. Sorrells, 1991. DNA markers in plant improvement. Advanced Agronomy, 46: 39-90.
- Khavkin, E.E., 1997. Molecular markers in plant cultivation. Agricultural Biology, 5: 3-21.
- Baranova, T.V., A.A. Shevunyaeva, A.M. Tishchenko and O.A. Ermakov, 2001. Enzymatic Activity of Medicinal Plants. Bulletin of TSU, 1: 24.
- Ivanova, D., O. Tasinov, D. Vankova and Y. Kiselova-Kaneva, 2011. Antioxidative potential of Agrimonia eupatoria L. Science Technologies. Vol. I, 1(Medicine): 20-24.
- Kubinova, R., D. Jankovska and V. Bauerova, 2012. Antioxidant and α-glycosidase inhibition and polyphenol content of five species of Agrimonia genus. Acta fytotechnicaetzootechnica, 2(Nitra, Slovaca Universitas Agriculturae Nitriae): 38-41.
- Menytsikova, E.B. and N.K. Zenkov, 1993. Antioxidants and inhibitors of radical oxidation processes. Successes of Modern Biology, Vol. 113, 4: 442-455.
- Peskan, T., M. Surkovic-Perica and M. Krsnik-Rasol, 1996. Biochemical characteristic of horse-radish tumour and teratoma tissues. Plant Physiology and Biochemistry, 34: 385-391.
- Kalieva, A.N. and G.U. Dyuskalieva, 2014. The study of anatomical and morphological features of Agrimonia asiatica Zus. vegetative organs growing in the South-East of Kazakhstan. Journal of PAE “Successes of Contemporary Science”, 9(part 2): 48-51. RINC impact factor 0.3. ISSN 1681-7494.
- Kalieva, A.N. and G.U. Dyuskalieva, 2014. Anatomical and morphological features of medicinal plants Agrimonia pilosa Ldb. growing in the protected areas of Kazakhstan. Bulletin of the KNU. Ecological series, 3(42): 174-179.
- Illustrative determinant of the plants of Kazakhstan, Vol.1, 1969. Almaty: Pubslishing House of Science.
- Prozina, M.N., 1960. Botanical microtechnic. Moscow: Pubslishing House of Graduate school, pp: 208.
- Flora of Kazakhstan (Vol. ІV), 1961. Almaty: Publishing House of the Academy of Sciences of the Kazakh SSR, pp: 480-481.

This work is licensed under a Creative Commons Attribution 4.0 International License.





