How to Cite | Publication History | PlumX Article Matrix
Benzene Adsorption on C24 Fullerene
Mohammad T. Baei1*, Masoumeh Moghimi2, Adel shojaei3
1Department of Chemistry, Azadshahr Branch, Islamic Azad University, Azadshahr, Golestan, Iran 2Department of Chemistry, Gonbad Kavoos Branch, Islamic Azad University, Gonbad Kavoos, Golestan, Iran 3Department of physics, Behbahan Branch, Islamic Azad University, Behbahan, Iran
ABSTRACT: Using density functional theory calculations, we investigated the benzene adsorption on the C24 fullerene. The Calculated adsorption energy of benzene on the C24 fullerene was about -2.93 and -3.20 kJ/mol. The results suggested that the C24 fullerene has low sensitivity to the presence of benzene and the electronic properties of the fullerene after benzene adsorption remain almost unchanged
KEYWORDS: C24 fullerene; Benzene; Adsorption; DFT study
Download this article as:| Copy the following to cite this article: Baei M. T, Moghimi M, Shojaei A. Benzene Adsorption on C24 Fullerene. Biosci Biotech Res Asia 2015;12(2) |
Introduction
Benzene is a colorless liquid with a sweet odor. It evaporates into the air very quickly and dissolves slightly in water. It is highly flammable and is formed from both natural processes and human activities. Benzene is widely used in the United States; it ranks in the top 20 chemicals for production volume. Some industries use benzene to make other chemicals which are used to make plastics, resins, and nylon and synthetic fibers. Benzene is also used to make some types of rubbers, lubricants, dyes, detergents, drugs, and pesticides. Natural sources of benzene include volcanoes and forest fires. Benzene is also a natural part of crude oil, gasoline, and cigarette smoke. Benzene increases the risk of cancer and other illnesses. Benzene is a notorious cause of bone marrow failure. Substantial quantities of epidemiologic, clinical, and laboratory data link benzene to aplastic anemia, acute leukemia, and bone marrow abnormalities. Therefore, adsorption and detection of benzene molecule is very importance.
After the synthesis of C60 fullerene [1], fullerenes have attracted great interest because of their physical and chemical properties and applications in nanomaterials [2] and biomedical science [3]. Also, they play a fundamental role in medical sciences, chemistry, biology, materials, electronics, and related fields [4–6]. Among the smaller fullerenes, C24 fullerene is a favorable candidate for examining in molecule electronic devices, nanotechnology, and biomedical engineering. Liang Xu et al. [7] have studied the interaction between empty C24 fullerene and the smallest amino acid (glycine). Their results showed that the glycine molecule is energetically favorable to interact on the C24 fullerene through the amino nitrogen active site. Also, orientation effect on the electronic transport properties of C24 fullerene were investigated by Wen-Kai Zhao et al. [8] between the electrodes (Au–C24–Au). Their findings showed the application of the C24 fullerene in the field of single molecular devices or nanometer electronics. On the other hand, there are few studies on the C24 fullerene and further study on the structures is of important tasks.
Computational methods
In this work, benzene adsorption on the C24 fullerene is considered. C24 fullerene with a D6d symmetric form consisting of two 6-membered rings joined by twelve 5-membered rings, are chosen for this purpose. We carried out calculations at the B3LYP [9- 10] level with the 6-31G*standard basis set. For the structures, the geometry optimization, natural bond orbital (NBO), density of states (DOS), energies, frontier molecular orbital (FMO), and frequencies were calculated. The adsorption energy (Ead) of benzene on the C24 fullerene is determined by:
Ead = Ebenzene/C24– [EC24 + Ebenzene] Eq. (1)
where Ebenzene/C24 is the adsorption energy of benzene on the surface of C24 fullerene. EC24 and Ebenzene are the total energy of the pristine C24 fullerene and benzene molecule. The negative values of Ead reveal that the adsorption is exothermic. The energy gap (Eg) of the optimized structures has been obtained by the energy difference between the highest occupied molecular orbital (HOMO) and the lowest un-occupied molecular orbital (LUMO). Also, Fermi level energy (EFL) and work function (Φ) of the considered structures are calculated. All calculations were performed for the structures by using the GAMESS suite of programs [11].
Results and discussion
The optimized structure of C24 fullerene with a D6d symmetric form (consisting of two 6-membered rings joined by twelve 5-membered rings) is shown in Fig. 1. The C-C bond lengths of pure C24 in the two 6-membered rings are 1.42 Å and the C-C bond lengths in the twelve 5-membered rings are in the range of 1.36-1.53 Å. The electronic property analysis based on DOS shows a HOMO-LUMO gap (Eg) of 1.82 eV for the fullerene that is very close to the results of Liang Xu et al [9] using the hybrid B3LYP functional with 6-31++G** basis set. The NBO charges on C atoms in the fullerene are from 0.015 to -0.015|e|, shows that not all the carbon atoms in the fullerene are neutral. The IR frequencies are in the range of 273.6-1563.2 cm−1; show that the structure is stable.
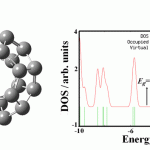 |
Figure 1: Optimized Structure of C24 fullerene and its density of states (DOS). |
First, we computed the optimized structures of the individual benzene molecule and C24 fullerene. Then, for investigation of benzene adsorption on the exterior surface of C24, various possible initial adsorption geometries of benzene molecule on the surface are considered. After full structural optimization without any constraints, the two most stable structures are obtained upon the relaxation processes which are shown in Figs. 2 and their electronic and thermodynamic properties are shown in Table1 and 2.
Table 1: adsorption energy of benzene on C24, Si@C24, Si-doped C24, and C20 fullerenes (Ead), HOMO energies (EHOMO), LUMO energies (ELUMO), HOMO–LUMO energy gap (Eg), Fermi level energy (EFL), and work function (Φ) for the studied systems.
| Structure | Ead(kJ/mol) | EHOMO(eV) | ELUMO(eV) | Eg(eV) | aΔEg(%) | bQT|e| | EFL(eV) | Φ(eV) | cΔΦ% |
| Benzene | – | -6.70 | 0.10 | 6.80 | – | – | -3.30 | 3.40 | – |
| C24 | – | -5.64 | -3.82 | 1.82 | – | – | -4.73 | 0.91 | – |
| Fig. 2A | -2.93 | -5.69 | -3.88 | 1.81 | 0.55 | -0.01 | -4.78 | 0.90 | 1.10 |
| Fig. 2B | -3.20 | -5.69 | -3.86 | 1.83 | 0.55 | -0.01 | -4.78 | 0.92 | 1.10 |
aThe change of Eg of C24 fullerene after benzene adsorption
bQT is defined as the total natural bond orbital charges on the benzene molecule
cThe change of work function of C24 fullerene upon benzene adsorption
In Configurations A and B in Fig. 2, the hydrogen atoms of benzene is interacted to 5 and 6-membered rings of the C atoms of the fullerene with the distance of about 3.10 and 3.26 Å, respectively. Calculated Ead values of the configurations are about -2.93 and -3.20 kJ/mol and a NBO charge of 0.01|e| is transferred from the fullerene to the benzene. The structural parameters of the configurations upon the adsorption process remain unchanged. The results show that the Configurations have a weak interaction between benzene molecule and the C24 fullerene. Also, in the configurations, the influence of benzene adsorption on the electronic properties of the fullerene was investigated. HOMO and LUMO energies, energy gap (Eg), Fermi level energy (EFL), and work function (Φ) of the configurations are given in Table 1. The EFL in a molecule is approximately middle of Eg and Φ for a semiconductor is defined as the energy difference between the EFL and the LUMO [12] which is important in field emission applications. The important sensing mechanisms in nanostructure devices is change of Eg the nanostructure and subsequently change of its conductivity upon the adsorption process [13]. Therefore, it is very important to calculate the DOS of the C24 fullerene in the configurations before and after benzene adsorption. DOS for the configurations is shown in Fig. 2. In comparison with the pristine model, their Eg values remain almost unchanged (changed by about 0.55 %). Also, for the configurations, Fermi level energy (EFL), and work function (Φ) values remain almost unchanged (changed by about 1.10 %). The results indicate that the benzene adsorption has no sensible effects on the electronic properties of the fullerene.
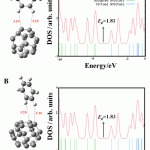 |
Figure 2: Adsorption configurations of benzene molecule and their density of states (DOS) on the C24 fullerene |
In the next step, the vibrational frequencies for the considered systems are calculated and are summarized in Table 2. The harmonic frequencies for the structures are negative, showing that the structures are unstable. Calculated values of ΔHad and ΔGad for the configurations show a physical adsorption between the benzene molecule and the C24 fullerene. The results show that C24 fullerenes cannot be used as a chemical adsorbent or sensor for benzene molecule.
Table 2: Calculated thermodynamic data [ΔH, ΔG (kcal/mol), and ΔS (cal/mol K)] and minimum and maximum vibrational frequencies (cm−1) for the studied systems.
| Structure | ΔHad | ΔGad | ΔSad | υmin | υmax |
| Benzene | – | – | – | 414.3 | 3214.4 |
| C24 | – | – | – | 273.6 | 1563.2 |
| Fig. 2A | -0.06 | 7.65 | -25.89 | -10.2 | 3209.8 |
| Fig. 2B | -0.04 | 8.35 | -28.14 | -8.6 | 3210.9 |
Conclusions
We investigated the benzene adsorption on C24 fullerene using DFT calculations. The results show that benzene molecule presents a weak physical adsorption with the fullerene. Also, the results suggest that the C24 fullerene has not sensitivity to the presence of benzene molecule. In total, the C24 fullerenes cannot be used as a chemical adsorbent or sensor for benzene molecule.
References
- H.W. Kroto, J.R. Heath, S.C. O’Brien, R.F. Curl, R.E. Smalley, C60: buckmin- sterfullerene, Nature (London) 318 (1985) 162.
- T. Akasaka, S. Nagase, A. New, Family of Carbon Clusters, Kluwer Academic Publisher, Dordrecht, The Netherlands, 2002.
- K. Muthukumar,J. A.Larsson, Explanation of the different preferential binding sites forCeandLainM2@C80(M¼Ce, La)–a density functional theory prediction, J.Mater.Chem.18(2008)3347.
- Yoon, M.; Yang, S.; Zhang, Z. J Chem Phys 2009, 131(6), 64707.
- Chamberlain, T. W.; Champness, N. R.; Schrder, M.; Khlobystov, A. N. Chem: Eur J 2011, 17, 668–674.
- Senapati, L.; Schrier, J.; Whaley, K. B. Nano Lett 2004, 4, 2073–2078.
- Liang Xu, Chao Li, Feng Li, Xiaojun Li, Shuqing Tao, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 98 (2012) 183–189.
- Wen-Kai Zhao,Chuan-Lu Yang, Jing-Fen Zhao, Mei-Shan Wang, Xiao-Guang Ma, Physica B 407 (2012) 2247–2253.
- Becke AD (1993) J Chem Phys 98:5648
- Lee C, Yang W, Parr RG (1988) Phys Rev 37:785
- Schmidt M, Baldridge K, Boatz J, Elbert S, Gordon M, Jensen J, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery Jr JA (1993) J Comput Chem 14:1347
- C. Kim, B. Kim, S.M. Lee, C. Jo, Y.H. Lee, Electronic structures of capped carbon nanotubes under electric fields, Phys. Rev. B. 65 (2002) 165418.
- X. Zhou, W.Q. Tian, X.-L. Wang, Adsorption sensitivity of Pd-doped SWCNTs to small gas molecules, Sensors and Actuators B: Chemical 151 (2010) 56–64.

This work is licensed under a Creative Commons Attribution 4.0 International License.





