Manuscript accepted on :
Published online on: 25-12-2015
Victor Aleksandrovich Stupin1 , Ekaterina Vladimirovna Silina2 , Rafael Gegamovich Oganov3 , Yevgeny Anatolevich Bogdanov4 and Natalia Nikolaevna Shusharina4
1Pirogov Russian National Research Medical University (RNRMU), Ostrovityanova str, 1, Moscow, 117997, Russia 2I.M. Sechenov First Moscow State Medical University (First MSMU), Trubetskaya str, 8, Moscow, 119991, Russia 3The National Research Center for Preventive Medicine, Petroverigsky str. – 10, Moscow, 101000, Russia. 4Immanuel Kant Baltic Federal University (IKBFU), Nevskogo Str., 14, Kaliningrad, 236041, Russia
ABSTRACT: The aim of the study is to develop an invasive device for long-term remote monitoring of cardiovascular system parameters, including blood pressure, in patients with comorbid conditions. Such a device is an important solution of a medical problem – continuous monitoring of patients with hypertension, arrhythmias, chronic heart failure and other comorbid vascular conditions.We developed a pilot model of a device for long-term remote monitoring of blood pressure (systolic, diastolic, mean), heart rate and other calculable parameters of cardiovascular system. We developed a model of a capacitive sensor based on a microelectromechanical system technology (MEMS), equipped with a continuously-adjustable capacitor, wireless data communication and electric power supply. Transmitter model is developed. Engineering tests of device prototype were performed. Experimental work demonstrating the feasibility of converting the collected signals into mm of Hg for the measurement of blood pressure changes and steady radiosignal transduction to the transmitter was performed.A variant of biocompatible cover was chosen – silicone and parylene C. The sensor is designed to be implanted into human or animal body and can be situated either in the vessel lumen or on the vessel wall.
KEYWORDS: microelectromechanical systems (MEMS); pressure sensor; blood pressure; medical invasive device
Download this article as:| Copy the following to cite this article: Stupin V. A, Silina E. V, Oganov R. G, Bogdanov Y. A, Shusharina N. N. Development of an Invasive Device for Long-Term Remote Monitoring of Cardiovascular System Parameters, Including Blood Pressure, in Patients with Comorbid Conditions. Biosci Biotech Res Asia 2015;12(2) |
Introduction
Medical devices are considered implantable if they are partially or completely inserted into the organism surgically or using other methods.Thanks to current advances in microelectronics, biotechnology and materials invasive medical devices are actively introduced into practice. At present 8-10% of population in USA and 5-6% of population of industrialized countries use implanted medical devices to improve quality of life and increase life expectancy [Jiang & Zhou, 2010].Since the first report about electrical stimulation of the heart in 1952[Zoll, 1952]to the first commercially available wireless blood pressure (BP) measurement system, introduced by Cardiomemsin 2011, specialists of different fields of science made significant efforts to improve patient quality of life using different medical devices, including implantable cardiac pacemakers, cochlear implants, implantable urinary bladder stimulators and implantable wireless BP sensors [Furman, 2002; Larsson et al., 2003; Mokwa, 2007; Wilson et al., 2008; Rajappan 2009; Axisa et al., 2009; Magjarevic et al., 2010; Beck et al., 2010; Majerus et al., 2011]. The most widely used sensors for the invasive measurement of different parameters of human body are devices, developed using microelectromechanical system (MEMS) technology, sized several micrometers to several millimeters, and combining mechanical and electric components in one microchip.
Recently many researchers focus on the development of implantable systems for real-time monitoring of vital signs, which can continuously work with signal pickup period less than 1 second [Narasimhan et al., 2010; Olivo et al., 2011; Bazaka et al., 2013]. This technology provides an opportunity to move from elimination of consequences of accomplished incidents and accidents to prevention. The main focuses of research are devices for long-term invasive BP monitoring [Silina et al., 2014].
Constant remote BP monitoring can be achieved by insertion of a sensor into the vessel lumen or onto the vessel wall. Each variant has its own advantages and disadvantages: insertion of a sensor into the vessel lumen provides more accurate data, but is associated with the danger of thrombus formation or active host immune reaction; sensor positioned on the vessel wall gives less accurate results and is associated with the possibility of rough connective tissue formation at the place of sensor localization.
Thus, at present there is a sensor for invasive BP monitoring (CardioMEMS Champion; США) sized 3,5х2х1,5 mm, which is approved for use in clinical practice. The device represents a sensor implanted into the pulmonary artery, with a radiofrequency antenna for wireless data transmission of blood pressure parameters. Anchor loops of the sensor are made of nitinol (nonmagnetic nickel-titanium alloy) and hold the device against the vessel wall, where it interferes with blood flow. Radiofrequency impulse of the sensor accumulates energy and after the termination of actuation the sensor transmits the signal onto the receiving antenna. Power supply of the device is provided via the external antenna, attached on the patient skin.The advantages of the device compared to other implantable hemodynamic monitors are easy and proved implantation technology, direct and accurate BP measurement, reduction of organism adverse reactions through the use of hypoallergenic steel of the device body and silicone covering, wireless data transmission, absence of implantable accumulator requiring replacement in the future. The main disadvantages of the device are the dependence on patient body position (data acquisition and transmission of BP measurement is performed only in recumbent position), temperature sensitivity (decrease of accuracy), inaccuracy in case of body weight change, dyspnoea etc., inability to register several parameters of cardiovascular system, risk of thrombosis[Scherr et al.,2009; Loh et al., 2013].
In 2014 were published the results of a prospective blind randomized clinical trial including 550 patients with III class cardiac failure, assigned to groups of treatment and control.CardioMEMS BP sensor was implanted into the pulmonary artery via the catheterization of right heart chambers. Follow-up period was 6 to 22 months. The hospitalization rate decreased by 50% after the mean of 17,6months in active treatment group (р<0,001). Constant remote control of central hemodynamics provided more effective and timely correction of diuretic and vasodilatory therapy [Adamson et al., 2014]. It is important to mention that in patients with intraarterial sensor treatment success was achieved regardless of ejection fraction level because of patient self-control and individualized therapy correction by attending physician.
Advanced development of medical devices.
At present scientists and development engineers focus on long-term functioning implantable BP sensors utilizing advanced biocompatible electronics and alternative energy sources. At present capacitive and piezoresistive sensors based on membrane technology for implanted BP monitoring systems, non-sensitive to mechanical fatigue and stiffness changes of the material are actively developed. Biocompatible electronics is actively developed, for instance, organic transistors [Yokota et al., 2013].
One of the main aspects of design is the covering of the device. MEMS are often covered with biocompatible polymers [Gutierrez& Meng, 2011].
At present there are hydrophilic, hydrophobic and biodegradable coatings. Hydrophilic coatings are unstable because of high water content and are inapplicable for the studied problem. The most promising for medical devices are hydrophobic coatings with high level of biocompatibility. The most suitable are parylene and silicone.
Clinical studies mainly concern silicone coatings. The first work was devoted to biocompatibility assessment of pressure sensor with 100 µm thick silicone coating (NUSIL MED-1511, Silicon Adhesive) in a mouse fibroblast culture L929 using standard MTT assay [Hierold et al., 1998]. The coating was not toxic for the cell type. The most cited is a paper published by a group of scientists from Case Western Reserve University, USA, who successfully performed a trial of silicone elastomer for medical purposes MDX4-4210 (Dow Corning Company, USA), applied onto the surface by simple dipping. Authors implanted a MEMS model for BP measurement into the carotid artery of a rat and demonstrated its safety, including thrombus formation[Cong P et al., 2006].Several laboratories in Belgian universities (University of Ghent, Katholieke Universiteit Leuven, University of Ghent) performed the trial of high-elasticity plasma-modified siliconeMDX-4210 (produced by Dow Corning, USA) [Axisa et al., 2009].Silicone was applied using biological adhesive NUSIL MED6-161. To enhance biocompatibility at the final stage sample surface was treated with plasma based on argon or oxygen, then the sample was treated with 2- acrylamide -2-methyl-propyl-sulfonate-Naand then with plasma once more which led to formation of graft-vinyl copolymers. Samples were sterilized using ethylene oxide and evaluated for cytotoxicity according to ISO 10993-1 (on fibroblasts). The evaluation demonstrated the absence of cytotoxicity. The only commercially available MEMS-device for the invasive BP measurement on the market, CardioMEMS, is hermetically covered in silicone. The device successfully passed the tests for efficacy and safety, including cytotoxicity, carcinogenic activity, sensitization, pyrogenicity and hemolysis. After the evaluation CardioMEMS was approved for clinical practice and was recommended by FDA [Loh et al., 2011;FDA and CardioMEMS, 2011]. The most recent preclinical randomized study of safety and efficacy of silicone coating for metal implanted devices is [Pavo et al., 2014]. The aim of the study was to evaluate the short-, middle-, and long-termresults of stents, implanted into coronary arteries of pigs with follow-up period up to 6 months. Study results demonstrated superiority of silicone-coated stents.
Parylene-based coating is thinner compared to silicone and is characterized by lower hydrophobic properties, which is an obvious advantage. However, preclinical and clinical studies, and also difficulties with surface application on the final product prevent this material from taking leading positions in the industry. At present parylene is widely used as an ultrathin medical stent coating.ParyleneНТ 197 nm thin was applied onto a MEMS-acoustic resonator with negligible resonance frequency changes [Clausen et al., 2010]. In 2014 researchers demonstrated significant advances in the evaluation of a telemetric stent for wireless intravessel pressure monitoring in pigs[Chen et al., 2010]. The evaluated stent has a spiral structure with an integrated microchip consisting of a capacitive pressure sensor and a high-performance inductor/stainless steel antenna. A 20-mm long device is covered with selective parylene C, generates an inductor-capacitor with resonance contour, which allows data transmission in the range 0-250 mm Hg in a radiofrequency range of 30-80MHz.
In the nearest future in is going to be possible to develop biodegradable sensors and batteries [Allen, 2014; Tsanget al., 2014], which make feasible the development of sensors with limited life period. The development of alternative energy sources instead of inductance coil comprises another field for the improvement of implanted pressure sensors. Transition from radiofrequency induction power supply to accumulation of energy in vivo can promote the development of integrated implant system [Fletter et al., 2009].
Therefore, the development of an invasive long-term cardiovascular remote monitoring system, including blood pressure monitoring, is of current interest, which substantiates the aim of the study.
Methods
During engineering work we utilized the following equipment: insertion soldering station JBC CD-2BB, dismantlement soldering station JBС DD-2B, with drying fan and control unit JBC JT-2A, hot pincer JBC MS-A, oscillograph Agilent DSO1002A, signal generator with variable waveform and frequency up to 20 MHz Agilent 33220A, digital multimeter Agilent 34410A, power supply module GInstek GPD-73303S.
Technical test methods of the device were performed the following way. The coil of wireless charging module «Seeeduino Wireless Charging Module» was placed not farther than 5 cm from the wireless charging module incorporated into the MEMS pilot device. Wireless charging module «Seeeduino Wireless Charging Module» was connected to power supply according to the developed circuit diagram. Then we turned the prototype device on (to current supply) and evaluated the current, then connected the output signal of the prototype to an oscillograph according to the scheme of technical tests. After that we started the evaluation of the variable capacitator capacity (during increase and decrease of capacity), which demonstrated the working efficiency of the device. After the tests current supply and oscillograph were turned off.
The evaluation of the feasibility of utilization of a capacitance pressure sensor was performed using an imitation of pressure change. Arterial pressure response was simulated by changing the variable capacity by several picofarad against the reference capacity. Time to threshold level of Schmitt circuit is directly proportional to capacitator capacity, therefore, time is different for a variable and reference capacitator, which allows to convert the signal into an impulse in sensor module in mm Hg.
Results.
Development and technical tests of the device for long-term invasive remote monitoring of cardiovascular system parameters.
A prototype of a device for invasive long-term remote monitoring of BP and other parameters of cardiovascular system was developed, consisting of an implanted MEMS-sensor, transmitter and programming unit.Prototype consists of two capacitive-temporal electrical circuits based on low-powered oscillators. The circuit provides the comparison of capacity of reference and variable capacitors. Power sources in two capacitive-temporal electrical circuits are selected to ensure similar current on both capacitors. Variable capacitor can have the same or greater capacity compared to reference capacitor, which results in a longer charging time.
The variable capacitor developed, which is used for MEMS pressure sensor simulation, has the following technical specifications: range of measured pressure 0,5–1,3 Bar, mean sensitivity – 0,451 picofarad/Bar, dimensions – 0,5х0,2 mm. Energy loss between the implant and the base station were determined according to Friis formula.
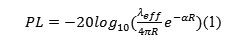
where α= |Im[k]|,λeff = 2π/Re[k] effective wavelength in lossy medium, k –complex wave number of mean loss and R – the distance between the receiving and transmitting antenna.
Developed prototype of capacitive MEMS-sensor is equipped with wireless data transfer system and wireless energy transmission system. The system consists of a capacitor of variable capacity with measured reference capacity 4 picofarad and a radiofrequency wireless data transfer module, intended for data transfer on a transmitter at 433 Mhz band. Wireless current supply is performed at 13,56 Mhz using a special module. Sensor interface is used to convert capacity values into a digital impulse signal.
Therefore, MEMS-sensor executes: 1) constant measuring and transformation of capacity value of a variable capacitor into a digital signal using a specialized module, 2) conversion of capacity value of variable capacitor into BP measurements, 3) transmission of data on an external device (transmitter) utilizing a single module of wireless energy transfer and data transmission.
A transmitter prototype is developed, consisting of a debug board of a microcontroller СС3200-LAUNCHXL (based on a system on a chip Wi-Fi + МК CC3200 with ARMCortexM4 architecture), display, prototype of wireless data and energy transfermodule, debug board GSM-module (SIM800H-EVM, permitting data transfer and patient geopositioning data, and receiving and transferring messages to a control microchip). Operation algorithm of debug board of microcontroller is realized on the base of real-time operating system for imbedded systems FreeRTOS. Resonant circuit of a transmitter operates as a series voltage resonance. Debug board of the transmitter forms signal at 10MHz frequency, which is amplified with current driver. Current driver provides a current on a resonant circuitup to 50 mAat the 5V voltage. Resonant circuit provides signal amplitude on a coil up to 100 V (at a tuned-circuit Q-factor – 20), which enables to transmit electric power on the MEMS-device prototype from a distance up to 30 cm. Data transmission is realized via amplitude modulation with modulation coefficient 100%. Maximal data transmission speed onto MEMS-sensor prototype – 20 kbit/sec. Data is received as follows:when data transmission from MEMS-sensor occurs, the level of signal on contour changes, converted analogue signal goes through a passive filter to separate “black” frequencies, then filtered signal goes to amplifier and to transmitter debug board.
Technical tests of the prototype were performed (30 tests). During the tests a standard 5V power source, digital multimeter and oscillograph were used. Signal from each of the two branches of capacity-temporal circuit represents a train of impulses; the frequency is proportional to 1/C. For valid signal extraction a logic element was used, which combines the signal with constant and variable frequencies into one as a train of impulses. Output signal was sent directly to microcontroller for subsequent processing. A variable capacitor was used as an active element, the change of capacity led to changes in the output signal recorded. Capacity value varied in the range of 5-30 picofarad. The value of capacity change of the output signal was changed at every stage manually by 0.5 – 1 picofarad. The results demonstrated a change of output signal proportionally to decrease of capacitor capacity. There was a clear relationship between the change of value of capacity of variable capacitor and output signal. Therefore we can postulate that data can be converted into the capacity value.
Experimental evaluation of the feasibility of the use of capacitance-type pressure sensor and wireless data and power transfer for invasive blood pressure monitoring.
An experiment aimed at demonstrating the change of signal length during simulated pressure change was performed. A prototype of BP sensor, connected to a power supply module via an inductor (output voltage 5V) was used for the experiment. For a temporary signal sweep a digital oscillograph was used. Capacity values in the range 4-20 picofarad were measured. Reference capacity value was 5 pF, the resulting oscillator frequency was 1 kHz. For the examination and test of capacity-temporal circuit the impulse was transmitted to the receiver. It was established that signal length changed in relation to capacitor capacity, so it can be converted into mm Hg for BP measurement.
The distance of steady signal transmission from a series of experiments was 10-12 cm. It is possible to increase the distance using an external antenna (this scenario was not implemented in the prototype due to the absence of necessity at that stage).
The test of feasibility of energy transmission using electric fields was performed. For that purpose a compact wireless charging module Seeeduino Wireless Charging Module, consisting of a transmitter and a coil was used. Steady work of prototype with the charging module through a sheet of paper was attained in experimental conditions. Physical parameters of paper (dielectric permeability, thickness) are similar to human skin. The experiment demonstrated steady energy transmission within a distance up to 2 cm.
We plan to perform experimental tests with MEMS-sensor, coated with biocompatible material, silicone for example, applied via dipping method.
Device implantation options.
Characteristic feature of proposed technical solution is the wireless energy transmission system (inductive method), presence of a signal processing module and several implantation options.
Several possible BP sensor implantation options in MEMS-device are considered.
Capacitive MEMS BP sensor is placed into a silicone cuff, filled with incompressible liquid. Silicone cuff is secured on the artery with a surgical suture Vicril 3.0, 4.0. The main module, consisting of wireless energy supply module and transmitter-receiver module, is placed into a silicone body and connected to BP sensor via conductors with electrical insulation (Figure 1).
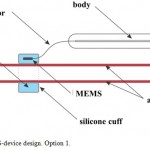 |
Figure 1: MEMS-device design. Option 1. |
Each of the proposed options has its advantages and disadvantages. The main advantage of option 1 is the possibility of implantation even in medical prevention centers without specialized vascular and/or catheterization laboratories; minimal possibility of thrombus formation because of the absence of manipulations on the inner artery wall; possibility of increasing sensor dimensions and registration of several physiologic parameters, not limited to BP, including heart functioning parameters, vessel wall stiffness with pulse wave velocity and other cardiovascular parameters. The main disadvantage of option 1 is the increase of error and the need for calibration of the device at least once a month, implantation of the sensor in typical places of BP measurement according to Korotkov’s method, namely only on the brachial artery, which has a diastolic diameter of 3.5 ± 0.1 mm in humans.
Capacitive MEMS BP sensor is placed on the artery wall, using a special mounting device (solitaire-type). In that case the power supply source must be placed into BP sensor body and equipped with wireless charging module. The main module, consisting of wireless energy and data transmission, is placed into a silicone body. Besides, the main module should be additionally equipped with wireless charging system of measuring part of the device;the sensor should contain a remote power supply source (Figure 2).
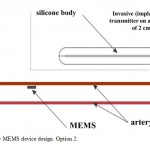 |
Figure 2: MEMS device design. Option 2. |
However, this engineering solution increases the risk of thrombus formation and BP sensor migration to distal parts of the vessel. This will inevitably result in full vessel obturation, which is similar to sensor diameter, so the signal will not only be lost as a result of critical distance between the sensor and receiver/transmitter exceeded, but also as a result of blood flow block and absence of signal per se. As a result, a special option (Option 3) for intravessel sensors was developed.
Capacitive MEMS BP sensor is positioned on an inner surface of arterial stent, which allows safe immobilization of the sensor with minimal vessel wall trauma. The stent is implanted into the artery using a standard X-ray surgery. In that case the stent attached to the BP sensor functions as an additional antenna, providing more clear BP signal registration. Besides, such technology permits stent implantation in any vessel, even in case of partial occlusion, with additional curative effect. As in the previous option, the main module contains wireless power supply and data transmission modules (Figure3).
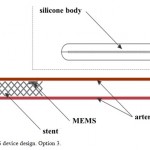 |
Figure 3: MEMS device design. Option 3. |
The main advantage of this option, besides the curative effect of stent implantation, is the accuracy of direct BP measurement, high level of safety and low risk of vessel thrombosis, which is a fundamental distinction of the developed devicefrom option 2. This option provides an opportunity of long-term remote BP monitoring in patients with chronic cardiovascular conditions. The implantation point depends on the vessel with medical indications for stenting.
Thus, the developed device has a wide range of opportunities for clinical implementation. The best option of extravessel implantation is Option 1 (Figure 1), for intravessel implantation – Option 3 (Figure 3).
Discussion
The role of hypertension as the main prognostic factor of cardiovascular accidents is obvious, which gives rise to substantial interest in the development of devices for constant remote BP monitoring in high-risk patients. The most recent engineering solutions worldwide for long-term monitoring of cardiovascular system parameters, mainly BP level, demonstrate the feasibility of medical and social demand implementation. That is why the development of an invasive device for long-term remote cardiovascular system monitoring, including BP, is highly relevant and promising. Such a device provides an opportunity for constant monitoring of patients with hypertension, arrhythmias, chronic heart failure and other comorbid cardiovascular conditions.
We developed a prototype of a device for long-term invasive remote BP and other cardiovascular parameters monitoring. A prototype of capacitive sensor, based on MEMS technology, equipped with a variable capacitor, wireless energy and data transmission module was developed. The sensor is designed for implantation into the human or animal body, can be positioned inside or outside of the vessel wall. Transmitter prototype is developed. Engineering tests of the device developed were performed. Experimental work demonstrated the feasibility of conversion of obtained signal into mm of Hg for BP measurement, and steady signal transmission. An option of biocompatible coating was chosen.
Conclusion
Experimental studies demonstrate the promising potential of the developed Russian prototype of the system with wireless energy supply and signal transduction. First successful prototype tests of a capacitive BP sensor, changing signal length via a simulated pressure change were performed. The results obtained permit to pass to the next stage of safety and efficacy trials on animal models. In the nearest future we plan to develop the draft design documentation and technical requirement for the implementation of research and development work for the realization of technology, enabling the most effective utilization of achieved results in the field of long-term BP monitoring in clinical practice with implantable biosensor systems. We also plan to further develop the method of digital conversion of biological signal and to develop general principles of remote monitoring and alerting about critical changes of cardiovascular system parameters in patients with vascular comorbidity.
We assume that such a device will perform real-time registration of information on human cardiovascular system performance. In case of critical BP changes alerting signals about the necessity of medical decisions are provided, including emergency medical service. This gives an opportunity to enhance the efficacy of preventive care and medical care in patients with cardiovascular pathology, increase life expectancy and decrease budget expenses, with no need for additional involvement of highly-skilled professionals in the program of follow-up care.
Acknowledgements
Authors express gratitude fora productive scientific co-operation to a number of research institutes and laboratories (Immanuel Kant Baltic Federal University, NSNUMoscow Engineering and Physics Institute, Moscow Aviation Institute, I.M. Sechenov First Moscow State Medical University, The National Research Center for Preventive Medicine, Russian Cardiology Research-and-Production Complex, MSU named after M.V. Lomonosov, NRC Kurchatov institute), a group of researchers for active contribution: Petrov V.A., Kasymov V.A., Shusharina N.N., Ladaniy D.V., Malkin M.N., Belyaeva N.N., RogozaA.N., Orlova A.S., OrlovV.A., Zolotariova L.S., Brilkov I.A.
Research was carried out with the support of Russian Foundation for Fundamental Research (RFFR), (project № 13-04-12087).
References
- Jiang, G., Zhou D.D.(2010) Technology advances and challenges in hermetic packaging for implantable medical devices. InD.D. Zhou, E.S. Greenbaum, editors. Implantable neural prostheses 2: techniques and engineering approaches (pp.28-61). Berlin: Springer.
- Zoll, P. M. (1952) Resuscitation of the heart in ventricular standstill by external electric stimulation. N Engl J Med 1952;247:768-71.
- Furman S. Early history of cardiac pacing and defibrillation. Indian Pacing Electrophysiol J 2002;2:2-3.
- Larsson B, Elmqvist H, Ryden L, Schuller H. Lessons from the first patient with an implanted pacemaker: 1958-2001. Pacing ClinElectrophysiol 2003;26(1 Pt 1):114-24.
- Mokwa W. Medical implants based on microsystems //Measurement Science and Technology. – 2007. – Т. 18. – №. 5. – С. R47.
- Wilson BS, Dorman MF. Cochlear implants: a remarkable past and a brilliant future. Hear Res 2008;242:3-21.
- Rajappan K. Permanent pacemaker implantation technique: part II. Heart 2009;95:334-42.
- Axisa F, Jourand P, Lippens E, Rymarczyk-Machal M, De Smet N, Schacht E, et al. Design and fabrication of a low cost implantable bladder pressure monitor. Conf Proc IEEE Eng Med Biol Soc 2009;2009:4864-7.
- Magjarevic R, Ferek-Petric B. Implantable cardiac pacemakers: 50 years from the first implantation. Zdrav Vestn 2010;79:55-67.
- Beck H, Boden WE, Patibandla S, Kireyev D, Gutpa V, Campagna F, et al. 50th Anniversary of the first successful permanent pacemaker implantation in the United States: historical review and future directions. Am J Cardiol 2010;106:810-8.
- Majerus SJ, Fletter PC, Damaser MS, Garverick SL. Low-power wireless micromanometer system for acute and chronic bladder-pressure monitoring. IEEE Trans Biomed Eng 2011;58:763-7.
- Narasimhan S, Wang X, Bhunia S. Implantable electronics: emerging design issues and an ultralight-weight security solution. Conf Proc IEEE Eng Med Biol Soc 2010;2010:6425-8.
- Olivo J, Carrara S, De Micheli G. Energy harvesting and remote powering for implantable biosensors. IEEE Sens J 2011;11:1573-86.
- Bazaka K, Jacob MV. Implantable devices: issues and challenges. Electronics 2013;2:1-34.
- Silina E.V., Stupin V.A., Kolesnikova E.A., Rumyantseva S.A., Oganov R.G. New devices for invasive blood pressure detection and the prototype for a long-term invasive distance control of cardiovascular system. Biology and Medicine, (2014) – 6(3), Article ID: BM-045-14, 5 pages.
- Scherr D., Kastner Р., Kollman A., Hallas A. et al. Effect of home-based telemonitoring using mobile phone technology on the outcome of heart failure patients after an episode of acute decompensation: randomized controlled trial //Journal of medical Internet research. – 2009. – 11(3):e34)/ doi:10.2196/jmir.1252 (http://www.jmir.org/2009/3/e34/(Data Views 23.05.2015)
- Loh J. P., Barbash I. M., Waksman R. Overview of the 2011 Food and Drug Administration Circulatory System Devices Panel of the Medical Devices Advisory Committee Meeting on the CardioMEMS Champion Heart Failure Monitoring System //Journal of the American College of Cardiology. – 2013. – Т. 61. – №. 15. – С. 1571-1576.
- Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, Henderson J, Cowart P, Stevenson LW. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction.Circ Heart Fail. 2014 Nov;7(6):935-44.
- Yokota, T.; Kuribara, K.; Tokuhara, T.; Zschieschang, U.; Klauk, H.; Takimiya, K.; Sadamitsu, Y.; Hamada, M.; Sekitani, T.; Someya, T. Flexible Low-Voltage Organic Transistors with High Thermal Stability at 250 °C. Adv. Mater. 2013, 25, 3639–3644.
- Gutierrez, C.A.; Meng, E. A Subnanowatt Microbubble Pressure Sensor Based on Electrochemical Impedance Transduction in a Flexible All-Parylene package. In Proceedings of the 24th International Conference on Micro Electro Mechanical Systems, Cancun, Mexico, 23–27 January 2011; pp. 549–552.
- Hierold C., B. Clasbrumme, D. Behrend, T. Scheiter, M. Steger, K. Oppermann, H. Kapels, E. Landgra, D. Wenze and D. Etzrodt, Implantable low power integrated pressure sensor system for minimal invasive telemetric patient monitoring, Micro Electro Mechanical Systems, MEMS, 1998. Proceedings, The Eleventh Annual International Workshop, 568 – 573/
- Cong P., Young D.J., Hoit B., Ko W.H. Novel long-term implantable blood pressure monitoring system with reduced baseline drift, EMBS. – 2006. -№06. – р. 1854–1857/
- Axisa F., Jourand P., Lippens E., Rymarczyk-Macha, M., de Smet N., Schacht E.,Vanfleteren J., Puers R., Cornelissen R. Design and Fabrication of a Low Cost Implantable Bladder Pressure Monitor. In Proceedings of the Annual International Conference of the IEEE on Engineering in Medicine and Biology Society, EMBC-2009, Minneapolis, MN, USA, 2–6 September 2009; р. 4864–4867/
- Loh JP, Barbash IM, Waksman R. Overview of the 2011 Food and Drug Administration Circulatory System Devices Panel of the Medical Devices Advisory Committee Meeting on the CardioMEMS Champion Heart Failure Monitoring System.J Am Coll Cardiol. 2013 Apr 16;61(15):1571-6;
- FDA and CardioMEMS Panel Package; Summary of safety and effectiveness data http://www.accessdata.fda.gov/cdrh_docs/pdf10/P100045b.pdf (Data Views 23.10.2014)
- Pavo N, Syeda B, Bernhart A, Szentirmai E, Hemetsberger R, Samaha E, Plass C, Zlabinger K, Pavo IJ, Petrasi Z, Petnehazy O, Hoerstrup SP, Maurer G, Gyöngyösi M. Preclinical randomised safety, efficacy and physiologic study of the silicon dioxide inert-coated Axetis and bare metal stent: short-, mid- and long-term outcome.EuroIntervention. 2014 Apr 29. pii: 20121003-0; http://www.ncbi.nlm.nih.gov/pubmed/24769439
- Clausen I., Seeberg T.M., Gheorghe C., Wang D.T. Biofouling on protective coatings for implantable MEMS. In Proceedings of 2010 IEEE Sensors, Kona, HI, USA, 1–4 Nov 2010. – p. 751–754.
- Chen X, Brox D, Assadsangabi B, Hsiang Y, Takahata K. Intelligent telemetric stent for wireless monitoring of intravascular pressure and its in vivo testing.BiomedMicrodevices. 2014 Oct; 16(5):745-59.
- Allen, M.G. Microfabricated implantable wireless microsystems: Permanent and biodegradable implementations. In Proceedings of the 2014 IEEE 27th International Conference on Micro Electro Mechanical Systems (MEMS), San Francisco, CA, USA, 26–30 January 2014; pp. 1–4.
- Tsang, M.; Armutlulu, A.; Martinez, A.; Herrault, F.; Allen, S.A.B.; Allen, M.G. A MEMS-enabled biodegradable battery for powering transient implantable devices. In Proceedings of the 2014 IEEE 27th International Conference on Micro Electro Mechanical Systems (MEMS), San Francisco, CA, USA, 26–30 January 2014; pp. 358–361.
- Fletter, P.C.; Majerus, S.; Cong, P.; Damaser, M.S.; Ko, W.H.; Young, D.J.; Garverick, S.L. Wireless micromanometer system for chronic bladder pressure monitoring. In Proceedings of the 2009 Sixth International Conference on Networked Sensing Systems (INSS), Pittsburgh, PA, USA, 17–19 June 2009; pp. 1–4.

This work is licensed under a Creative Commons Attribution 4.0 International License.





