How to Cite | Publication History | PlumX Article Matrix
Effects of LED Irradiation on the Growth and Astaxanthin Production of Haematococcus Lacustris
Hai-Linh Tran, Kyu-Han Lee and Chang-Hee Hong
LED Agri-bio Fusion Technology Research Center, Chonbuk National University, Iksan Campus, South Korea
ABSTRACT: Microalgae have long been considered promising microorganisms, due to their potential as a source of valuable pharmaceuticals, pigments, carbohydrates, biofuels, and other fine chemicals. High-density microalgal photobioreactors have been considered for exploiting the biotechnological potential of microalgae. Haematococcus lacustris is considered the richest natural source of astaxanthin for pharmaceutical, nutraceutical, and cosmetic applications. One of key challenges was to provide light sources that are more efficient, to deliver light more efficiently to the microalgal culture. Recent developments in highly efficient light-emitting diodes (LEDs) have made possible the development of LED-based photobioreactors (PBR). We investigated the effects of various LEDs on the growth and astaxanthin production from Haematococcus lacustris. The mixture of red and blue was found to be the most effective light source for growing this microalga. Different wavelength mixing ratios of red and blue were also investigated. The ratio of mixed red–blue of 1:3 showed the best growth and astaxanthin accumulation from Haematococcus lacustris. The maximum biomass and astaxanthin production with illumination by mixed red–blue LEDs at a ratio of 1:3 obtained was 2.3 g/L and 55.1 mg/L, respectively. Biomass and astaxanthin were enhanced by a maximum of 3.28 g/L and 84.12 mg/L, with the mixed red–blue illumination in the ratio 1:3 at high light intensity 160 µE· m-2 · s-1.
KEYWORDS: Astaxanthin; Haematococcus lacustris; light emitting diodes; microalga; wavelength
Download this article as:| Copy the following to cite this article: Tran HL, Lee KH, Hong CH. Effects of LED Irradiation on the Growth and Astaxanthin Production of Haematococcus Lacustris. Biosci Biotech Res Asia 2015;12(2) |
Introduction
Microalgae have been suggested as good candidates for fuel production because of specific advantages they possess such as higher photosynthetic efficiency, higher biomass production and faster growth when compared with other energy crops1. Microalgae have been considered as great promising microorganism due to its potential as a source of valuable pharmaceuticals, pigments, carbohydrates, biofuels and other fine chemicals. Haematococcus lacustris is green microalga, considered as the best potential source of astaxanthin. Astaxanthin (3,3′-dihydroxy-β, β-carotene-4,4′-dione) is a high value carotenoid pigment with potential applications in various fields, including cosmetics, nutraceuticals, and the food and feed industries2. The potent antioxidant properties of astaxanthin have been implicated in various biological activities, demonstrated in both experimental animals and clinical studies. It has both considerable potential and promising applications in human health and nutrition3. This photosynthetic microorganism requires different culture conditions for vegetative growth and astaxanthin production; astaxanthin accumulates inside Haematococcus cell bodies during the transformation of green vegetative cells to red cyst cells under unfavorable environments4. Many induction methods, such as nitrogen starvation, exposure to intense light, excess acetate addition, salt stress, and the addition of specific cell division inhibitors, have been developed for transforming green cells to red cysts with high astaxanthin content5. Accordingly, the biotechnological exploitation for growth and astaxanthin production potential of microalga requires exploration.
The most relevant environmental factors that affect the growth of microalgae include light, temperature, pH, salinity, nutrients’ qualitative and quantitative profiles, and dissolved oxygen, and the levels of heavy metals or synthetic organics6. Microalgal growth can be affected by reactor operation conditions such as hydraulic residence time, harvesting rates, gas transfer, and mixing equipment, because these factors affect CO2 availability, shear rates, and light exposure6. However, although work suggests that nitrogen is a critical factor, it does not work alone, but interacts with other factors such as the light intensity to affect the overall astaxanthin production. The effect of light intensity on astaxanthin production can be largely altered by the algal density in the culture, as the latter affects the light availability for individual cells under a given incident light intensity7. The light regime inside a cultivation system must be considered in the design and scaling-up of photobioreactors, because light energy is a growth limiting substrate. Two properties of light energy are important for astaxanthin accumulation: Light intensity and spectral quality8. A high light intensity causes relatively large quantities of astaxanthin to accumulate in Haematococcus cells. Spectral quality is defined by the absorption spectrum for chlorophylls and other photosynthetically active pigments (such as astaxanthin and carotenoids)9; supplying a specific wavelength of light will enhance astaxanthin production. Light emitting diodes (LEDs) have recently been recognized as effective light sources and low cost energy sources for microalgae cultivation10. For these reasons, it is important to develop general knowledge in this field, with an emphasis on the effects of light emitting diodes.
In the present study, the growth and astaxanthin accumulation of Haematococcus lacustris was examined for various light sources. A mixture of red and blue was found to be the most effective light source for growing this microalga, and different wavelength mixing ratios of red and blue were investigated. A 1:3 ratio of mixed red–blue light was found to be the most efficient for growth and astaxanthin production in Haematococcus lacustris. Herein, Haematococcus lacustris biomass was observed as a potential source of astaxanthin production when cultured using light emitting diodes.
Materials and Methods
Microalgal strain and culture systems
Haematococcus lacustris was purchased from the Culture Collection of Alga at the University of Texas at Austin. It was cultivated photoautotrophically in the OHM medium, which consisted of (g/L) KNO3, 0.41; Na2HPO4, 0.03; MgSO4·7H2O, 0.246; CaCl2·2H2O, 0.11; and (mg/L) C6H5FeO7, 2.62; CoCl2.6H2O, 0.011; CuSO4.5H2O, 0.012; MnCl2.4H2O, 0.98, Cr2O3, 0.075; Na2MoO4.2H2O, 0.12; SeO2, 0.005 and in (µg/L) biotin, 25; thiamine, 17.5 and B12, 15 11. Flask photobioreactors were made of Duran glass, and air that had been sterilized by filtering through glass fiber was passed into the flasks12. Fluorescent lamps were used as a light source for the seed culture, and the cultivations were maintained at 25 °C.
Biomass estimation
Aliquots of the culture suspension were harvested via centrifugation at 5,000 rpm for 10 minutes, after which the cells were washed twice with distilled water. The dry weight of the algal cells was then measured by pipetting the washed cell suspension onto pre-weighed Nylon filters (Model R04SP0470S, GE Osmonics Labstore, Minnetonka, MN, USA). After applying a vacuum from a small compressor for 5 minutes, the liquid component of the samples had been substantially removed, leaving the cell pellet. The filter and cell pellet were then placed in the drying oven and dried to constant weight by heating at 80 °C for 24 hours. The filters were then allowed to cool to room temperature in a desiccator and reweighed, after which the dry weight of the algal biomass was determined gravimetrically as the difference between the weight of the filter before and after use; growth was expressed in terms of dry weight (g/L)13.
Astaxanthin measurement
The astaxanthin concentration was analyzed using a spectrophotometer after acetone extraction together with bead-beating using a cell disruptor. The extraction was centrifuged at 13,000 rpm for 10 minutes, determined by the spectrophotometer at OD474, and calculated using calibration curves. The astaxanthin standard (Sigma-Aldrich Co. Ltd, U.S.A.) was used for calibration. The level of astaxanthin production (mg/L) (CA) was calculated using the following equation:
CA= (OD474-0.0831)/0.1426
Light sources and measurement of light intensity
Different light sources were used: Fluorescent lamps (Model FPL-55W, OSRAM Co., Ltd., Korea), blue (λmax 447 nm) and red LEDs (λmax 656 nm) were made by LED Agri-Bio Fusion Technology Research Center, Chonbuk National University, South Korea. The light intensities of the fluorescent lights were adjusted by altering the number of fluorescent lamps and the distance from the photobioreactor. The light intensity of the LED light was controlled using a light controller, thus the light intensities of the LEDs could be adjusted. The light intensities were measured using a quantum sensor (Model SKP 200 38684, Skye Instruments Ltd, United Kingdom).
Table 1: Mixing ratios of red and blue LEDs
|
Light intensity |
Wavelength mixing ratio | |
| Red light (wavelength range 595–700 nm, with a peak at 656 nm) | Blue light (wavelength range 400–505 nm, with a peak at 447 nm) | |
|
40 μE·m-2·s-1 |
2 | 2 |
| 1 | 3 | |
| 3 | 1 | |
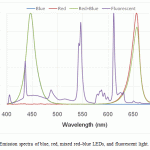 |
Figure 1: Emission spectra of blue, red, mixed red–blue LEDs, and fluorescent light |
 |
Figure 2: Schematic diagram of the experimental setup for different light illuminations (A: Fluorescent light, B: Red LED, C: Blue LED, D: A mixture of red and blue LEDs). |
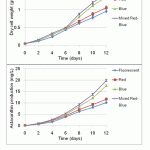 |
Figure 3: Biomass concentration and astaxanthin production of H. lacustris under various light sources |
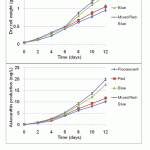 |
Figure 4: Biomass concentration and astaxanthin production of H. lacustris under different ratios of mixed red and blue LEDs |
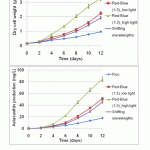 |
Figure 5: Effect of shifting wavelength and light intensity of LEDs on biomass concentration and astaxanthin production of H. lacustris |
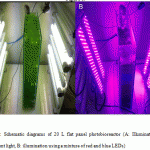 |
Figure 6: Schematic diagrams of 20 L flat panel photobioreactor (A: Illumination using fluorescent light, B: illumination using a mixture of red and blue LEDs) |
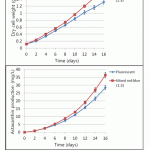 |
Figure 7: The growth and astaxanthin accumulation of H. lacustris in large-scale cultivation using a flat panel photobioreactor. |
Results and Discussion
Effects of different kinds of LEDs on the growth and astaxanthin production
In this study, we took steps to determine whether growth and astanxanthin production were affected by wavelength. We used the same experimental setting, except using different light sources such as fluorescent lighting, red, blue, and mixed red–blue LEDs. LEDs were used because it is possible to control their wavelengths precisely. Blue light (wavelength range 400–505 nm, with a peak at 447 nm) and red light (wavelength range 595–700 nm, with a peak at 656 nm) were used14. Fluorescent light was used as the control. As shown in Figure 1, the emission spectra of these light sources are very distinctive from one another. The cultures were illuminated at the same total light intensity (40 μE·m-2·s-1), and the pre-culture of H. lacustris was mixed in OHM medium in a 500 mL Duran flask, with a working volume of 350 mL. Inorganic carbon for microalgae growth was supplied at 1 vvm (aeration volume/medium working volume/minute) using an air pump and air sparger. The schematic diagram of the experimental setup is shown in Figure 2. As seen in Figure 3, the mixed–blue LED setup was found to be the most effective for growth of the microalga. The maximum biomass obtained with mixed red–blue illumination was 1.48 g/L, followed by blue, red, and fluorescent light at 1.36, 1.06, and 0.96 g/L, respectively. Compared with monochromatic light, the growth and astaxanthin production of H. lacustris using mixed red–blue LEDs were substantially higher. These results indicate that mixed LEDs are more favorable and effective for biomass and astaxanthin production in H. lacustris.
Effects of different mixture ratios of red and blue lights on growth and astaxanthin production
To evaluate the growth and astanxanthin production by wavelength mixing ratio based on light intensity, blue light (400–505 nm, with a peak at 447 nm) and red light (595–700 nm, with a peak at 656nm) were used14. The evaluated mixing ratios of red and blue light are presented in Table 1. The batches were cultured for 18 days, and all other operating conditions were the same as the previous experiment. As seen in Figure 4, a 1:3 mixture of red and blue light was found to be the most effective for growth and astaxanthin production in this microalga. The maximum biomass of 2.3 g/L was obtained with illumination by mixed red–blue light at a ratio of 1:3, followed by the ratios of 2:2 and 3:1, which yielded 2.08 and 1.76 g/L, respectively (Figure 4). The biomass production rate of ratio 1:3 light was approximately 10 and 20% higher than ratios 2:2 and 3.1, respectively. Confirmed results were observed in the level of astaxanthin production. The maximum astaxanthin levels of mixed red–blue light (ratio 1:3) was 55.1 mg/L at day 18, 50.3 mg/L for ratio 2:2 light, and 36.3 mg/L for ratio 3:1 light, respectively (Figure 4). Ruyters reported that sufficient red and blue light should be provided for the adequate photosynthesis of microalgae and plants15. In this study, photosynthetic efficiency increased by selectively and simultaneously providing both red and blue light, which provided the necessary wavelength ranges for photosynthesis. Thereby, the biomass and astaxanthin production rates were higher than for only red light, only blue light, or fluorescent light.
Effects of shifting the wavelength and initial light intensity of mixed red–blue LEDs on growth and astaxanthin production
To examine the effect of light intensity and wavelength of blue, red and mixed red–blue light on growth and astaxanthin production further, H. lacustris cells were cultured under various conditions. One at low light intensity (40 μE·m-2·s-1), one at high light intensity (160 μE·m-2·s-1) of mixed red and blue wavelengths (ratio 1:3), and one experienced shifting wavelengths (red light for four initial days, then mixed red–blue (ratio 1:3) for four days, then blue light for four days) with light intensity at 40 μE·m-2·s-1. The batches were all cultured for 12 days in total. Fluorescent light was used as a control (at 40 μE·m-2·s-1 light intensity). Figure 5 shows that the mixture of red and blue light (ratio 1:3) at a high light intensity (160 μE·m-2·s-1) was the most effective for growth and astaxanthin production. It displayed the highest biomass (3.28 g/L) and astaxanthin accumulation (84.12 mg/L), followed by the sample of mixed red–blue light (ratio 1:3) at low light intensity (40 μE·m-2·s-1) with 2.24 g/L and 54.54 mg/L. The next highest was shifting wavelength (2.12 g/L and 45.42 mg/L), then fluorescent (0.96 g/L and 17.86 mg/L). These results suggest that red–blue light, whose wavelength falls on the adsorption spectra of astaxanthin and chlorophylls, has higher efficiency than non-overlapping spectral sources. Furthermore, low light intensities (40 μE·m-2·s-1) were insufficient to support high-density photoautotrophic cultures or fully induce astaxanthin. However, the efficiency of high light intensities of mixed red–blue (160 μE·m-2·s-1 at a 1:3 ratio) have been jumped recently. This clearly suggests that LEDs can be suitable light sources, not only for biomass, but also for astaxanthin accumulation.
Validation of the growth and astaxanthin accumulation of H. lacustris in large-scale cultivation using a flat panel photobioreactor
Bubble column, semi-permeable membrane, tubular and flat panel photobioreactors have been scaled up to large-scale, yet neither has been demonstrated as clearly superior to the other16,17,18. The flat panel photobioreactor used in this experiment is a transparent vessel (made of glass) in which mixing is carried out directly in the reactor via air sparging. This photobioreactor was made with height 60 cm, width 45 cm, and thickness 8 cm, and its total working volume is 20 L. In this kind of photobioreactor, light dilution is obtained by applying a larger specific surface and self-shading to the panels. In this manner, it is possible to achieve higher photosynthetic efficiency. It was experimentally characterized for developing scalable industrial photobioreactors with LED illumination. The schematic diagrams of the flat panel photobioreactor are described in Figure 6. H. lacustris cells were cultured in flat panel photobioreactors under mixed red–blue illumination (at a ratio of 1:3) and a fluorescent light was used as a control at the same light intensity (40 μE·m-2·s-1). As seen in Figure 7, the biomass and astaxanthin production under the illumination of mixed red–blue light (1.6 g/L and 36.44 mg/L, respectively) was higher than for fluorescent light (1.32 g/L and 28.45 mg/L, respectively). It was confirmed that LEDs can improve the quality and quantity of microalgal biomass and their integration into microalgal production systems (photobioreactors) should be considered.
Acknowledgements
This work was supported by the Industrial Technology Research Infrastructure Program (N0000004) funded by the Ministry of Knowledge Economy (MKE, Korea).
References
- Tran H-L, Kwon J-S, Kim ZH, Oh Y, and Lee C-G. Statistical optimization of culture media for growth and lipid production of Botryococcus braunii LB572. Biotechnol. Bioprocess Eng., 2010; 15: 277-284.
- Katsumata T, Ishibashi T, and Kyle D. A sub-chronic toxicity evaluation of a natural astaxanthin-rich carotenoid extract of Paracoccus carotinifaciens in rats. Toxicology Reports., 2014; 1: 582-588.
- Guerin M, Huntley ME, and Olaizola M. Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol., 2003; 21: 210-216.
- Kang CD and Sim SJ. Selective Extraction of Free Astaxanthin from Haematococcus Culture Using a Tandem Organic Solvent System. Biotechnol Progr., 2007; 23: 866-871.
- Fábregas J, Domínguez A, Álvarez DG, Lamela T, and Otero A. Induction of astaxanthin accumulation by nitrogen and magnesium deficiencies in Haematococcus pluvialis. Biotechnol Lett., 1998; 20: 623-626.
- Kumar A, Ergas S, Yuan X, Sahu A, Zhang Q, Dewulf J, et al. Enhanced CO2 fixation and biofuel production via microalgae: recent developments and future directions. Trends Biotechnol., 2010; 28: 371-380.
- Wang J, Sommerfeld MR, Lu C, and Qiang Hu. Combined effect of initial biomass density and nitrogen concentration on growth and astaxanthin production of Haematococcus pluvialis (Chlorophyta) in outdoor cultivation. Algae., 2013; 28: 193-202.
- Kim N-J and Lee C-G. A theoretical consideration on oxygen production rate in microalgal cultures. Biotechnol. Bioprocess Eng., 2001; 6: 352-358.
- Kim T-H, Lee Y, Han S-H, and Hwang S-J. The effects of wavelength and wavelength mixing ratios on microalgae growth and nitrogen, phosphorus removal using Scenedesmus sp. for wastewater treatment. Bioresource Technol., 2013; 130: 75-80.
- Schulze PSC, Barreira LA, Pereira HGC, Perales JA, and Varela JCS. Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol., 2014; 32: 422-430.
- Fábregas J, Domínguez A, Regueiro M, Maseda A, and Otero A. Optimization of culture medium for the continuous cultivation of the microalga Haematococcus pluvialis. Appl Microbiol Biotechnol., 2000; 53: 530-535.
- Tran H-L, Hong S-J, and Lee C-G. Evaluation of extraction methods for recovery of fatty acids from Botryococcus braunii LB 572 and Synechocystis sp. PCC 6803. Biotechnol. Bioprocess Eng., 2009; 14: 187-192.
- Tran H-L, Ryu Y-J, Seong D, Lim S-M, and Lee C-G. An effective acid catalyst for biodiesel production from impure raw feedstocks. Biotechnol. Bioprocess Eng., 2013; 18: 242-247.
- Tran H-L, Lee K-H and Hong C-H. Influence of light emitting diodes on the growth and astaxanthin production of Haematococcus pluvialis. J. Bio Inno., 2015; 4(3): 92-101.
- Ruyters G. 1984. Effects of blue light on enzymes. In: Senger, H. (Ed.), Blue Light Effects in Biological Systems. Springer Verlag, Berlin. 283-301.
- Tran H-L, Kwon J-S., and Lee C-G. Optimization for the growth and the lipid productivity of Botryococcus braunii LB572. J. Biosci. Bioeng., 2009; 108: S55.
- Acién Fernández FG, Fernández Sevilla JM, and Molina Grima E. Photobioreactors for the production of microalgae. Reviews in Environmental Science and Bio/Technology. 2013; 12: 131-151.
- Kim Z-H, Park H-W, Ryu Y-J, Shin D-W, Hong S-J, Tran H-L, Lim S-M, Lee C-G. Algal biomass and biodiesel production by utilizing the nutrients dissolved in seawater using semi-permeable membrane photobioreactors. J. Appl. Phycol., 2015; 1-11.

This work is licensed under a Creative Commons Attribution 4.0 International License.





