How to Cite | Publication History | PlumX Article Matrix
Efficacy of Sphaeranthus Indicus in the Treatment of Rifampicin Induced Hepatotoxicity in Rats.
Devendra S. Shirode1*, Brijendra B. Jain2, C. B. Mahendra Kumar3,
1Dept. of Pharmacology, Padm. Dr. D. Y. Patil College of Pharmacy, Akurdi, Pune – 44 and Jawaharlal Nehru Technological University (JNTU), Hyderabad, Telangana - 500 085 2Associate Director, YSPM’s Yashoda Technical Campus, Satara, Maharashtra, India, 3St. Mary’s College of Pharmacy, St. Francis Street, Secunderabad – 500 025
ABSTRACT: The present investigation was aimed to study the effect of ethanol extract of Sphaeranthus indicus against rifampicin induced hepatotoxicity in rats. The 70 % ethanol extract of aerial part of Sphaeranthus indicus (SIEE) at the doses of 200 and 400 mg/kg and silymarin 100 mg/kg were administered to the rifampicin challenged rats. The effect of SIEE and silymarin on physical (wet liver weight, liver volume) and biochemical parameters (SGOT, SGPT, ALP, direct and total Bilirubin) were measured in rifampicin induced hepatotoxicity in rats. Similarly, hepatic tissues were subjected to histopathological observations and in-vivo antioxidant activity (tissue glutathione and lipid peroxidation levels). Treatment with SIEE (200mg/kg and 400mg/kg) reduced the elevated levels of above mentioned physical parameters and biochemical markers of hepatotoxicity. Histopathological findings and in vivo antioxidants studies also confirmed that SIEE possess hepatoprotective effects. The hepatoprotective and in vivo antioxidant properties may be attributed to the polyphenolic compounds like flavonoids, saponins and tannins that are present in the SIEE.
KEYWORDS: Sphaeranthus indicus; hepatoprotective; in vivo antioxidant; Rifampicin
Download this article as:| Copy the following to cite this article: Shirode D. S, Jain B. B, Kumar C. B. M. Efficacy of Sphaeranthus Indicus in the Treatment of Rifampicin Induced Hepatotoxicity in Rats. Biosci Biotech Res Asia 2015;12(2) |
Introduction
Rifampicin is mainly used anti-tuberculosis drug, but it causes liver damage by induction of cytochrome P 450 (Yew and Leung, 2006). Conventional or synthetic drugs for treatment of liver diseases are sometimes inadequate and can have serious adverse effects. In view to develop potent hepatoprotective agent from natural source against rifampicin induced hepatotoxicity in albino rats, the efficacy of Sphaeranthus indicus was investigated in rats.
Sphaeranthus indicus Linn belongs to family Asteraceae. The plant is reportedly used for treating epileptic convulsions, mental illnesses and hemicranias (Kirtikar and Basu, 2003). It is used as a tonic, laxative, digestive, anthelmintic and in the treatment of insanity, tuberculosis, diseases of the spleen, anemia, bronchitis, elephantiasis, pain of the uterus and vagina, piles, asthma, leucoderma and hemicranias (Kirtikar and Basu, 2003; Paranjape, 2001). Literature reports of this plant revealed the presence of an essential oil, glycosides and eudesmanoids (Balsas, 1959), an alkaloid sphaeranthine (Basu et. al. 1946) and an isoflavone 5,4’-dimethoxy-3’ –prenylbichanin 7-0-β-galactoside with some interesting sesquiterpine (Gupta et. al., 1967; Gogate et. al., 1986; Shekhani et. al., 1990 ) and a new flavone glycoside (Yadav et. al., 1998).
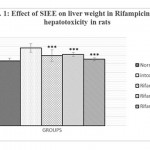 |
Figure 1 |
The pharmacological data reveals that plant possess analgesic activity (Nanda et. al., 2009), anticonvulsant activity (Sander et. al., 1996; Harbone, 1998), hepatoprotective activity (Tiwari et. al., 2009; Nayak et. al., 2007), anthelmintic activity (Lal et. al., 1976), antimicrobial activity (Sohoni et. al., 1988, ), antihyperlipidemic (Pande and Dubey, 2009), anti-inflammatory (Jain et. al., 2003; Heinrich et. al., 1998), antioxidant (Tiwari et. al., 2009, Shiwarkar et. al., 2006), anti-diabetic (Prabhu et. al., 2008) and neuroleptic activity (Galani and Patel, 2009).
It is claimed that flowers of Sphaeranthus indicus has hepatoprotective activity against acetaminophen induced hepatotoxicity in rats. However hepatoprotective activity of other parts of the herb has not been investigated for hepatoprotective activity. In this study we have attempted to investigate the hepatoprotective activity of aerial parts of Sphaeranthus indicus in rifampicin induced liver damage in rats.
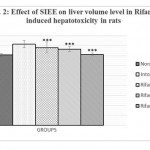 |
Figure 2 |
Materials and Methods
The collected aerial part of Sphaeranthus indicus were air dried and powdered. The powder of Sphaeranthus indicus was extracted with petroleum ether, chloroform and 70 % ethanol in a Soxhlet extractor. The mark was removed by filtration and the extract evaporated to dryness at a low temperature under reduced pressure in a rotary evaporator. Preliminary phytochemical screening of ethanol extract of aerial part of Sphaeranthus indicus (SIEE) revealed the presence of saponins, glycosides, flavonoids, tannins, steroids and proteins.
Animals
Wistar albino rats (150-220g) and mice (18-25 g) of either sex were used for the study. Approval from the institutional animal Ethical committee (1555/PO/a/11/CPCSEA) for usage of animal in the experiment was obtained as per the Indian CPCSEA guidelines.
Acute Toxicity Studies
The acute toxicity was determined on albino mice by fixed dose method of OECD Guide line no 420 given by CPCSEA. The acute toxicity studies revealed that the ethanol extract is safe at 2000 mg/kg. Hence extract was treated as non-toxic and 1/10th (200mg/kg) and 1/5th (400mg/kg) of the 2000 mg/kg was selected for hepatoprotective activity.
Experimental Designs
(Bafna et. al., 2004; Kulkarni et. al., 2007)
The animals were divided into five groups of six animals each.
Group I: Normal control group – The animals in this group received distilled water (1 ml/100 gm, p.o.) as vehicle in 4 divided doses.
Group II: Positive control (toxic) group – The animals in this group received rifampicin (1 gm/kg, p.o. in 5 % gum acacia) at day one.
Group III: Standard group – The animals in this group received rifampicin (1 gm/kg, p.o. in 5 % gum acacia) at day one, and after 30 min. Silymarin (100 mg/kg, p.o.) was administrated in four divided doses.
Group IV: SIEE group – The animals in this group received rifampicin (1 gm/kg, p.o. in 5 % gum acacia) at day one, after 30 min. SIEE (200 mg/kg p.o.) was administered in four divided doses.
Group V: SIEE Group – The animals in this group received rifampicin (1 gm/kg, p.o. in 5 % gum acacia) at day one, after 30 min. SIEE (400 mg/kg p.o.) was administered in four divided doses.
On 2nd day, after overnight fasting, all the animals were anesthetized with anesthetic ether and blood was withdrawn by retro-orbital plexus method. Collected blood was centrifuged (2000 rpm for 10 mins) to get clear serum and various biochemical parameters like SGPT (Bradley et al., 2003), SGOT (Rej et al., 1973), ALP (McComb et al., 1972), Bilirubin (total and direct), (Pearlman et al., 1974) were estimated.
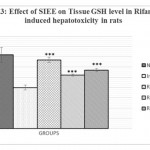 |
Figure 3 |
The liver was dissected out and processed for glutathione (GSH), LPO, wet liver weight, liver volume and histopathological investigations.
In vivo tissue GSH estimation
Tissue Glutathione (GSH) measurements were performed using the modification of Ellamn procedure (Aykae et. al., 1985). Liver tissue samples were homogenized in ice cold trichloroacetic acid (1gm tissue in 10 ml 10% TCA) in an ultra trux tissue homogenizer. The mixture was centrifuged at 3000 rpm for 10 mins. Then 0.5 ml of supernatant was added to 2ml of (0.3M) disodium hydrogen phosphate solution. A 0.2 ml solution of dithiobisnitrobenzoate (0.4mg/ml in 1% sodium acetate) was added and absorbance was measured at 412 nm.
In vivo lipid peroxidation estimation
The degree of lipid peroxide formation was assayed by monitoring thiobarbituric reactive substance formation (Buege et al., 1978). Take 1.0 ml of biological sample (0.1-2.0 mg of membrane protein or 0.1-2.0 μmol of lipid phosphate) to this add 2.0 ml of TCA-TBA-HCL solution and mixed thoroughly. Solution was heated for 1 hr and cooled. Then precipitate was removed by centrifugation at 1000 rpm for 10 min. and absorbance of sample was determined at 535 nm against a blank that contain all the reagents minus lipid.
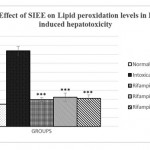 |
Figure 4 |
Statistical Analysis
Results were expressed as mean ± SEM (n=6). Statistical analysis was performed with one way ANOVA followed by Turkey-Kramer multiple comparison test. P values less than 0.05 was considered to be statistically significant (p<0.05).
Results and Discussion
Rifampicin is rapidly metabolized to deacetyl rifampicin, which binds to RNA polymerase and it leads to inhibition of RNA synthesis; thus inhibiting the nucleic acid and protein synthesis. It induces fatty liver and finally cirrhosis. This causes fatal liver damage acute hepatic failure, which is accompanied by increase in the activity of some serum enzymes (Bafna and Mishra, 2004; Shankar et. al., 2005).
In the rifampicin induced liver damage in albino rats, the increased levels of SGOT and SGPT in serum are indicative of cellular leakage and loss of functional integrity of cell membrane in liver (Drotman and Lowhorn, 1978). Alkaline phosphatase (ALP) is the prototype of these enzymes that reflect the pathological alteration in biliary flow (Ploa and Hewitt, 1989). ALP is membrane bound protein enzyme, which are released unequally depending on the pathological phenomenon (Szezeklik et. al., 1961). Rifampicin induced elevation in this enzymatic activity in serum is in line with high level of serum bilirubin content.
Animals treated with only rifampicin showed a significant (p˂0.01) increase in the level of biochemical parameters (SGPT, SGOT, ALP and Bilirubin), physical parameters (wet liver weight, liver volume), liver peroxide content (LPO) and a significantly (p˂0.01) decrease tissue glutathione (GSH), when compared with normal group of animals. Treatment with SIEE showed significant (p˂0.01) improvement in liver damage in all above parameters in dose dependent manner (Table 1). Histopathological studies also confirmed these findings (figure 5).
The hepatoprotective activity of SIEE against rifampicin induced liver damage may be due to inhibitory effects on formation of the active metabolic, 25-desacetyl rifampicin which in turn reduces drug metabolizing enzymes. The SIEE showed significant (p˂0.01) reduction in biochemical markers, which suggest the stability of the biliary functions during injury and it indicate the sign of regeneration process of liver.
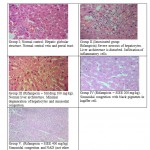 |
Figure 5: Histopathological studies |
It can be concluded from this study that, administration of SIEE minimize the effect of rifampicin induced liver damage in rats. In addition, the hepatoprotective property may be attributed to the polyphenolic compounds present in the plant extract, namely tannins, flavonoids and saponins. Further investigation is going on to isolate, characterize and screen the active principles that possess antioxidant and hepatoprotective property in the plant extract.
Table1 : Effect of SIEE on Biochemical markers in rifampcin induced hepatotoxicity
| Treatment | Biochemical parameters Mean ± SEM | ||||
| SGOT
U/L |
SGPT
U/L |
ALP
IU/L |
Total Bilirubin
mg/dl |
Direct Bilirubin
mg/dl |
|
| Normal control
(1ml dist. water p.o.) |
90 ±
5.10 |
59 ±
5.26 |
116.5 ±
13.576 |
0.62 ±
0.026 |
0.19 ±
0.019 |
| Positive (toxic) control (1ml dist.water p.o.+ Rifampicin 1 gm/kg p.o.) | 619 ±
29.57 |
693 ±
79.38 |
680.17 ±
24.898 |
2.56 ±
0.216 |
1.485 ±
0.06 |
| Rifampicin + Silymarin
(1 gm/kg p.o.+ 100mg/kg, p.o.) |
145±
6.875*** |
110±
7.30*** |
190.16±
7.298*** |
0.728±
0.037*** |
0.2±
0.018*** |
| Rifampicin + SIEE (1 gm/kg p.o.+ 200mg/kg p.o.) | 427±
14.631*** |
323.5±
13.114*** |
514±
23.88*** |
0.795±
0.042*** |
0.228±
0.015*** |
| Rifampicin +SIEE (1 gm/kg p.o.+ 400mg/kg p.o.) | 290±
16.310*** |
191.66±
15.43*** |
478.5±
21.81*** |
0.715±
0.0619*** |
0.205±
0.017*** |
Values are the mean ± S.E.M. of six rats/ treatment.
Significance ***P<0.001 and **P<0.01, compared to positive (toxic) control treatment
Acknowledgements
The authors are thankful to Padmashree Dr. D. Y. Patil college of Pharmacy, Akurdi, Pune for providing all the facilities to carry out this research work. We are grateful and thank PARDO preclinical research and development organization Pvt. Ltd. Pune for expert opinion in his to pathological studies.
Reference
- Yew, W.W., Leung, C.C. Anti-tuberculosis drugs and hepatotoxicity. Respirology, 2006; 11: 1599-604.
- Kritikar, K.R., Basu, B.D. Indian Medicinal Pants, lalit mohan basu, Allahabad: 2003; pp 1347.
- Paranjape, P., Indian Medicinal Pants, In: Forgetten healer: A guide of herbal medicine. Delhi: Chaukhambba Sanskrit pratisthan; 2001; pp 148-149.
- Baslas, K. Essential oil from Sphaeranthus indicus, Perf. Ess. oil Rec., 1959; 50:765.
- Basu, N.K., Lamsal, P.P. Chemical Investigation of Sphaeranthus indicus. Linn. J. Am. Pharm. Asso., 1946; 35: 274-5.
- Gupta, R.K., Chandra, S., Mahadevan, V. Chemical composition of Sphaeranthus indicus. Linn. Indian J Pharm., 1967; 29: 47-8.
- Gogate, M.G., Ananthasubramanian, L., Nargund, K.S., Bhattacharya, S.C. Some interesting sesqueterpenoids from Sphaeranthus indicus Linn. Indian J. Chem., 1986; 25: 233-8.
- Shekhani, M.S., Shah, P.M., Yasmin, A., Siddiqui, R., Perveen, S., Khan, K.M. An immunostimulant sesquiterpene glycoside from Sphaeranthus indicus. Phytochem., 1990; 29: 2573–6.
- Yadav, R.N., Kumar, S. 7-hydroxy-3’, 4’, 5, 6-tetramethoxyflavone, A new flavone glycoside from the stem of Sphaeranthus indicus Linn. J. Inst. Chem., 1998; 70: 164-6.
- Nanda, B.K., Jena, J., Rath, B., Behera. B.R. Analgesic and antipyretic activity of whole parts of Sphaeranthus indicus Linn. J. Chem. Pharm. Res., 2009; 1: 207-12.
- Sander, J.W.A.S., Shrovon, S.D. Eipdermiology of epilepsies, J. Neurolo. Neurosurg. Psychiatry, 1996; 61, 433-43.
- Harborne, J.B. Phytochemical Methods: Guide to Modern Techniques of plant Analysis, 3rd edition, New Delhi: Springer, Rajkamal Electric press, 1998; I-32, 40-227.
- Tiwari, B.k., Khosa, R.L. Hepatoprotective and antioxidant effects of Sphaeranthus indicus against acetaminophen induced hepatotoxicity in rats. J. Pharma. Sci. Res., 2009; 1: 26-30.
- Nayak, S.S., Maity, T.K., Maiti B.C. Hepatoprotective activity of Sphaeranthus indicus Linn. Int. J. Green Pharm., 2007; 1: 32–6.
- Lal, J., Chandra, S., Raviprakash, V., Sabi, M. In vitro anthelmintic action of some indigenous medicinal plants on Ascardia galli worms, Indian. J. Physiol. Pharmacol., 1976; 20(2): 64-8.
- Sohoni, J.S., Rojatkar, S.R., Kulkarni, M.M., Dhaneshwar, N.N., Tavale, S.S., Gururow TN. A new eudesmenolide and 2-hydroxycostic acid from Sphaeranthus indicus Linn. X-ray molecular-structure of 4α, 5α-epoxy-7α-hydroxyeudesmanolide. J. Chem. Soc. Perkin, 1988; 1: 157-60.
- Pande, V.V., Dubey, S. Antihyperlipidemic activity of Sphaeranthus indicus on atherogenic diet induced hyperlipidemia in rats. Int. J. Green. Pharm., 2009; 3: 159-61.
- Jain, A., Basal, E. Inhibition of Propionibacterium acnes-induced mediators of inflammation by Indian herbs. Phytomedicine, 2003; 10: 34–8.
- Heinrich, M., Robles, M., West, J.E., Ortiz, D., Montellano, B. R., Rodriguez, E. Ethnopharmacology of Mexican Asteraceae (Compositae). Annu. Rev. Pharmacol. Toxicol., 1998; 38: 539–65.
- Shirwaikar, A., Prabhu, K.S., Punitha, I.S. In vitro antioxidant studies of Sphaeranthus indicus (Linn.). Indian J. Exp. Biol., 2006; 44: 993-6.
- Prabhu, K. S., Lobo, R., Shirwaikar, A. Antidiabetic properties of the alcoholic extract of Sphaeranthus indicus in streptozotocin-nicotinamide diabetic rats. J. Pharm. Pharmacol., 2008; 60: 909-16.
- Galani, V.J., Patel, B.G. Effect of hydroalcoholic extract of Sphaeranthus indicus against experimentally induced anxiety, depression and convulsions in rodents. Int. J. Ayurveda Res., 2010; 1: 87-92.
- Bafna, A.R., Mishra, S. H. Effect of methanolic extract of Achyranthes aspera Linn. On rifampicin induced hepatotoxicity in rats. Ars. Pharm., 2004; 45(4): 343-51.
- Kulkarni, A.S., Siraskar, B.D., Deshpande, A.D., Kulkarni, A.V., Dhounde S.M., Study of hepatoprotective activity of Portulaca oleracea L. on rifampicin induced hepatotoxicity in rat. The pharmacist, 2007; 2 (2): 1-5.
- Bradley, D.W., Maynard, J.E., Emery, G., Webster, H. Transminase activity in serum of long term hemolysis patients. Clin. Chem., 2003; 18: 1442.
- Rej, R., Fasce, C.F., Vanderlinde, R.E. Increased aspartase aminotransferase activity of serum after in vitro supplymentation with pyridoxal phosphate. Clin. Chem., 1973; 19: 92.
- McComb, R.B. Bowers, G.N. Study of optimum buffer conditions for measuring alkaline phosphatase activity in human serum. Clin. Chem., 1972; 18: 97.
- Pearlman, P.C., Lee, R.T. Detection of measurement of total Bilirubin in serum with use of surfactants as solubilizing agents. Clin. Chem., 1974; 20: 447.
- Aykae, G. M., Vysal, A.S., Yalein, T.N., Kocak, A. S. Oz, H. The effect of chronic ethanol ingestion on hepatic lipid peroxide, Glutathione, glutathione peroxidase and glutathione transferase in rats. Toxicology, 1985; 36: 71-6.
- Buege, A. J., Steven, A.D. Microsomal lipid peroxidation. London: Moury Kleiman Co. 1978; 302.
- Shankar, M.B., Parikh, J.R., Geetha, M., Mehta, R.S., Saulija, A.K. Hepatoprotective activity of a benzopyrone from Tephrosia purpurea pers. Journal of natural remedies. 2005; 5 (2): 115-20.
- Drotman, R.B., Lowhorn, G.T. Serum enzymes as indicators of chemical induced liver damage. Drug Chemical Toxicology, 1978; 1: 163–71.
- Ploa, G.L., Hewitt, W.R. Detection and evaluation of chemically induced liver injury. In: Wallace Hayes, A. (Ed.), Principles and Methods of Toxicology, 2nd ed. Raven Press, New York 1989; pp. 399–628.
- Szczeklik, E., Orlowski, M., Szewes, A., Serum gamma glutamyl peptidase activity in liver disease. Gastroenterology, 1961; 41: 353-9.

This work is licensed under a Creative Commons Attribution 4.0 International License.





