How to Cite | Publication History | PlumX Article Matrix
Identification of the Carriers of Genes for Resistance to Wheat Leaf Rust Using Molecular Markers
A. Madenova1, A. Kokhmetova2, G. Kampitova2 , M. Atishova2, L. Purnhauser3
1Kazakh National Agricultural University, Kazakhstan, 050010, Almaty, Abay ave. 8 2Institute of Plant Biology and Biotechnology,Kazakhstan, 050040, Almaty, Timiryazev Str 45 3Cereal Research Non-Profit Ltd. Co., Hungary, H-6726, Szeged, Alsókikötő-sor 9
ABSTRACT: Wheat leaf rust is an important disease of wheat, which causes economic damage to the country. Epiphytotic diseases of wheat rust cover the entire continents leading to catastrophic crop failures. In order to control the stability, it is very important to have available molecular genetic markers linked to these symptoms. As a result of the phytopathological evaluation of susceptibility to rust on the background of infectious diseases, we selected a number of samples resistant to Puccinia recondite f. sp. tritici using the following molecular markers: F1.2245/Lr10-6/r2, csLV34, LN2/Ventriup and csGS-F/R. We identified 20 samples resistant to wheat stem rust. From the studied wheat material we identified nine samples with Lr10 gene, one with Lr34/Yr18 genes, 2 with complex genes Lr37/Sr38/Yr17 and 10 samples with Lr68 genes. The most valuable donor of sustainability is a promising line Almaly/Obriy, where 3 resistance genes Lr34/Yr18, Lr37/Sr38/Yr17, Lr68 and Yr2 were identified as well as Oktyabrina line with 2 resistance genes - Lr10 and Lr68. Our results provide an opportunity to move the selection process in Kazakhstan to a new scientific level by using molecular genetic techniques and technologies of MAS-breeding.
KEYWORDS: wheat resistant genes; rust leaf; molecular markers
Download this article as:| Copy the following to cite this article: Madenova A, Kokhmetova A, Kampitova G, Atishova M, Purnhauser L. Identification of the Carriers of Genes for Resistance to Wheat Leaf Rust Using Molecular Markers. Biosci Biotech Res Asia 2015;12(2) |
Introduction
Wheat is one of the most important crops in the world. It is the staple food for 35% of the population and provides about 20% of the calories consumed on the planet (Morgounov A., 2012). One of the main reasons for shortage of the crop in Kazakhstan includes diseases caused by a droplet infection. Rust diseases of wheat are one of the main reasons for the decrease in wheat crops. In the history of agriculture there are epiphytotic diseases of wheat rust covering the entire continents. This often led to catastrophic crop failures (Koishibayev M., 2002). Economic losses from pathogens and parasites significantly affect the production of wheat. Leaf rust is one of the most common diseases. During the human history it often was the reason for hunger and destruction of the economy of entire countries (Agrios G., 2005). Currently, worldwide annual losses from leaf rust are estimated at 2 billion USA dollars in equivalent (Bange G.A., 2013).
According to the experts of the Food and Agriculture Organization (FAO) of the United Nations Organization, annual global crop losses from parasites and diseases of agricultural plants had grown from 52.2 million conventional grain units in 1986-1990 up to 70 mln. tons in 1998-2005. A similar trend towards the increase in their severity and injuriousness is expected in Kazakhstan. About 15.5 million hectares of cereal crops are sowed in Kazakhstan. They produce about 17-18 million tons of grain. Approximately 8 million tons of grain is exported to Europe, Middle East and Arab countries. At the same time the loss of the wheat crop in the country differ from the disease and has reached 25 to 30% in recent years. Crop loss is an economic factor. It greatly affects the stable development of agricultural production (Kohmetova A.M. et al., 2014).
FAO and BGRI experts are working hard on the global program against the wheat rust, while providing political, financial and technical assistance to the interested world countries including CIS countries. Preventive measures are the issue of the special interest. These measures include: creation, removal and cultivation of new wheat varieties resistant to rusts, training of farmers, use of certified seeds, germplasm exchange, enhancing of monitoring and response in emergency situations, as well as international co-operation. In order to reduce the risk of possible types of epiphytotic rust diseases, Kazakh scientists are working in collaboration with CIMMYT and ICARDA (Morgounov A.I. et al., 2006).
Wheat rust diseases are the most common and devastating diseases of wheat. Leaf rust pathogen (the pathogen is Puccinia recondita Rob. ex. Desm. f. sp. tritici Erikss et Henn) poses a risk to wheat during the growing season of plants in the time period from seedlings to maturation of wheat. The main factors that determine the degree of development of leaf rust are the temperature, temperature of urediniospores germination from 2 to 32 °C with an optimum range at 15-20 °C and the presence of moisture drip for at least 4-6 hours (Lebedev V.B. et al., 1994). In general, crop losses from leaf rust depend on the intensity of the disease and on the duration of primary infection by leaf rust fungi. Upon the occurrence of favorable conditions for leaf rust agents, yield loss can reach 45% (Terekhov V.I. et al., 1982). In case of infection persistence in the earing stage, the rate of the disease can reach up to 80-100%. At this time damage to crops can be about 50% (Tansky V.I. et al., 1998).
In the scientific sources there is a wealth of information on the genes of wheat resistance to leaf rust pathogen Puccinia recondita Rob. ex Desm. f. sp. tritici. To date in Catalogue of gene symbols for wheat (gene catalogue of McIntosh et al., 2008) there is information on 67 Lr-genes. 63 of which are dominant, 4 (Lr30 and Lr37, Lr48, LrVPM) – recessive, and 2 (Lr27 and Lr31) – complementary ones. However, one of the main problems of short effectiveness of Lr-genes is the emergence of virulent races of the pathogen that are able to overcome the resistance. As a result, many of the known resistance Lr-genes became ineffective (McIntosh R.A. et al., 2010).
Methods and materials
Molecular Materials
The Lr10 gene is localized on chromosome 1AS and its sources are the wheat cultivars Lee and Timstein (McIntosh R.A. et al., 1995); the isogenic line RL6004 is a testing line for this gene (Choudhuri, H.C., 1958). It encodes such protein as CC-NBS-LRR with N-terminal domain. Upon the expression in transgenic wheat plants, Lr10 provides increased resistance to leaf rust. The Lr34 gene is localized on the chromosome 7D, the isogenic line RL6058 is a testing line for this gene (Dyck, P.L., 1987). A small group of genes for resistance to leaf rust (such as Lr34 and Lr46) is known as «slow rusting genes» (Singh R.P. et al., 2003). They provide long-term and nonspecific resistance of adult plants. However, their effect is more limited than that of race-specific genes. Lr34 has recently been cloned (Krattinger S.G. et al., 2009) and it has been shown that it relates to the family of Yr18 gene (gene of resistance to yellow rust of adult plants), genes of resistance to powdery mildew (Pm38) and leaf necrosis (Ltn1). Lr37 gene is localized on chromosome 2AS (Bariana H.S., McIntosh R.A., 1993); this gene was transferred to common wheat from Aegilops ventricosa Tausch., the isogenic line RL6081 is a testing line for this gene (Roelfs A.P. et al., 1992). It is available in Madsen, Rendezvous, VPM1 breeds. Carriers of this gene are affected in the juvenile phase. However they show an age resistance (Maia N., 1967). Long chromosomal fragment (25-38 cM) containing three genes of rust resistance was translocated between the short arms of 2NS chromosomes Triticum ventricosum and 2AS wheat chromosome. This gene is tightly linked to genes responsible for resistance to stem (Sr38), and yellow (Yr17) rust (McIntosh R.A. et al., 2010). The Lr68 gene is localized on chromosome 7BL (Herrera-Foessel S.A. et al., 2009). The common wheat cultivar Parula possesses a high level of slow rusting, adult plant resistance (APR) to all three rust diseases of wheat, including Lr68 gene (Herrera-Foessel S.A. et al., 2012). This cultivar can be used as a positive control for the Lr68 gene. We discovered molecular markers flanking Lr68 gene which can be used in marker selection. Parula breed was created by CIMMYT scientists in 1981. It also combines APR-resistance genes such as Lr34 and Lr46 (William H.M. et al., 1997; William H.M. et al., 2007; Herrera-Foessel S.A. et al., 2009). Perhaps the origin of the Lr68 gene is a Brazilian breed Frontana (Herrera-Foessel S.A. et al., 2012).
The number of effective Lr-resistance genes to the agents of leaf rust reduced every year. A constant search for such genes is required. They are relevant and important for breeding. Molecular markers may be advantageously used in the selection process.
The development of molecular markers linked to the APR genes of long-term resistance (that are effective for selection) is an issue of special importance. Specific markers for complex genes Lr34/Yr18/Pm38, Lr46/Yr29, Sr2/Yr30 and Lr67/Yr46 were created. These provide resistance to two or three diseases (Lagudah E.S. et al., 2006; Singh R.P. et al., 1998; Spielmeyer W. et al., 2003; Dyck P.L., Samborski D.J.; 1979).
Molecular markers may be successfully used in the selection process. In the present study the attention was drawn to the part of the effective genes for leaf rust resistance – Lr10, Lr68 and complex genes Lr34/Yr18 and Lr37/Sr38/Yr17, which were identified during molecular screening of wheat germplasm.
Basic research methods
The objects of the study were 35 samples taken from wheat breeding farm in 2013-2014. Resistance analysis of the samples was carried out at the field hospital of the Kazakh Institute of Agriculture and Crop Production. Phytopathological evaluation of rust resistance of experimental wheat material was performed in natural conditions and on the background of infectious diseases by the method of R.A. McIntosh et al., 1995 (McIntosh et al., 1995). According to this method, we established the percentage of infection rate and the type of infectious disease (0 – immune, R – resistant, MR – moderately resistant, MS – moderately susceptible, S – susceptible). The breed Steklovidnaya-24 was used as a sensitive local standard. An isolation of genomic DNA from the plant material was carried out from wheat seedlings using STAV (with 5-day-old seedlings) (Riede, CR, Anderson, JA 1996). In order to identify the carriers of resistance genes we conducted PCR (polymerase chain reaction) analysis with specific primers paired with genes of wheat leaf rust resistance. We used wheat isogenic lines and samples as a positive control with identified resistance genes. The volume of the reaction mixture for PCR was 25 µL, containing 2.5 µL of 10X buffer with Taq-polymerase, 2.5 µL of dNTP, 0.5L of each primer, 0.5 µL of Taq-polymerase, and 18 µL of MQ-H2O. For the separation of the amplified DNA fragments, we carried out electrophoresis in 2% agarose gel in TBE buffer (45 mM tris-borate, 1 mM EDTA, pH 8) (Chen XM et al., 1998). Amplification was performed in a thermocycler BIORAD (T100 TM Thermal Cycler, USA) with the following parameters: initial denaturation – 94 ºC for 5 min; 45 cycles – 1 min at 94 ºC; 1 min – 45 ºC; 2 min – 72 ºC; final elongation was performed for 7 minutes at 72 ºC.
Results and discussion
To date the selection using DNA technology is one of the most important methods to improve the efficiency of the selection process. Identification of genes in various MAS (Marker assisted selection) schemes can significantly reduce the size of the sample compared with conventional breeding methods. Using MAS technology also help to decrease the time of backcrosses and to control the size of the foreign fragment (Timonova E.M. et al., 2013). The present study is based on molecular screening of wheat samples for the presence of Lr-resistance genes. In samples we identified effective genes against leaf rust: Lr10, Lr68, Lr34/Yr18 and Lr37/Sr38/Yr17.
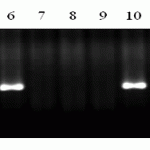
In order to identify Lr10 gene, we used marker F1.2245/Lr10-6/r2. Primer sequence F1.2245/Lr10-6/r2 (F: GTGTAATGCATGCAGGTTCC, R: AGGTGTGAGTGAGTTATGTT) (Schachermayr G. Et al., 1997), and an expected fragment of amplification had the size if 310bp. Figure 1 shows an electrophoretogram of DNA amplification products. We used isogenic line Thatcher Lr10 TC * 6/Exchange (RL6004) as a positive control.
 |
Click here to view full figure |
M- molecular weight marker (Gene Ruler 100 bp DNA Ladder), 1 Almaly/Umanka/1, 2 Almaly/GF70/1, 3-Naz/Obriy/1, 4-Naz/GF55/1, 5 Naz/GF55/4, 6- Almaly/5347Opata85/3, 7-425/GF55/1 8-425/GF55/2, 9-428g/MK-122/2, 10-Bermet/RWKLDN9, 11-BDME/Yr2, 12-Sanzar/RWKLDN9/2 13 Almaly/Obriy, 14-Madsen, 15-Almaly/5242Oxley1, 16-23/Kupava/1, 17 – Negative control (ddH2O), 18-Lr10 TC * 6/Exchange (RL6004) (positive control).
Picture 1. Products of DNA amplification of wheat samples (SP2) using primers to the locus F1.2245/Lr10-6/r2 resistance genes Lr10.
PCR product typical to gene carriers Lr10 had the size of 310bp in 3 samples of wheat Almaly/5347Opata85/3, Bermet/RWKLDN9, Madsen (Figure 1). Evaluation on the background of infectious diseases to the Kazakh population of brown rust showed moderate stability of these wheat samples (5MR-30MR).
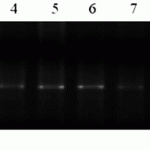
The gene of leaf rust resistance Lr34 is one of the effective genes meshed with the gene of resistance to yellow rust Yr18, marker csLV34 (F: GTTGGTTAAGACTGGTGATG, R: TGCTTGCTATTGCTGAATAGT), an expected fragment size 150bp. In order to identify carriers of Lr34/Yr18 genes, we performed PCR with primers to STS-locus csLV34. The last is a bi-allelic locus located at the distance of 0.4 cM from the Lr34 gene (Lagudah ES et al., 2006). We used isogenic lines of the breed Thatcher (RL6058) as a positive control and breed Anza in order to identify resistance to Lr34 gene (Figure 2).
 |
Click here to view full figure |
M- molecular weight marker (Gene Ruler 100 bp DNA Ladder), 1-Lr34 TC * 6/PI58548 (RL6058) positive control, 2-Anza, positive control, 3-Almaly/GF70/1, 4-Naz/Obriy/1, 5-Naz/GF55/1 6- Naz/GF55/4 7-425/GF55/1, 8-Almaly/Obriy, 9-425/GF55/2, 10-428g/MK-122/2, 11-Bermet/RWKLDN9, 12- Bermet/MK3797/1.
Figure 2. Molecular screening of constant forms (SP2) for the presence of complex of genes Lr34/Yr18, 2% agarose gel.
Thus, during the molecular screening we identified only one source of Lr34/Yr18 gene – line (Almaly/Obryi). Evaluation of the resistance of the Kazakh population of brown rust showed high resistance (0-5R).
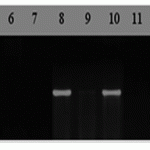
In order to identify carriers of complex genes Lr37/Sr38/Yr17, we performed PCR amplification using CAPS markers. CAPS-marker LN2/Ventriup (primers: LN2 5’AGGGGCTACTGACCAAGGCT-3′ and 5‘-TGCAGCTACAGCAGTATGTACACAAAASTS-3’) in the present time is one of the most popular marker for screening wheat all over the world. Molecular weight of the control amplification product with LN2/Ventriup marker is 262bp (Helguera M. Et al., 2003). We used American breed Madsen as a positive control in order to identify resistance gene Lr37 (Figure 3).
 |
Click here to view full figure |
M – molecular weight marker (Gene-Ruler 50bp DNA Ladder); Almaly/Umanka/1, 2 Almaly/GF70/1, 3-Naz/Obriy/1, 4-Naz/GF55/1, 5 Naz/GF55/4 6-428/Umanka, 7-425/GF55/1, 8-L372 Almaty polukarlikovaya/Progress, 9-428g/MK-122/2, 10-Bermet/RWKLDN9, 11-BDME/Yr2, 12-Sanzar/RWKLDN9/2, 13-Almaly (225)/5347Opata85, 14 -Bermet/MK3797/1, 15 Almaly/Obriy, 16-23/Kupava/1, 17 -Avoset/Naz, 18- Madsen, positive control, 19 – negative control (ddH2O). 2% agarose gel.
Figure 3. DNA amplification products of wheat samples (SP2) using primers to the locus CAPS-LN/VENTRIUP of linked with a complex of resistance genes Lr37/Sr38/Yr17.
Analysis of PCR results showed that 3 genotypes developed amplification product similar to Lr37/Sr38/Yr17 gene marker. Carriers of this gene include Almaly line/Obriy, L372 Almaty semi-dwarf/Progress, Madsen (Figure 3). Estimation on the background of infectious diseases to the Kazakh population of brown rust showed moderate resistance (5R-5MR).
In order to identify carriers of Lr68 gene, we used PCR amplification using STS primers csGS-F/R (Herrera-Foessel SA et al., 2009; (Herrera-Foessel SA et al., 2012). The gene Lr68 is a gene of age stability (APR). It provides sustained development of wheat leaf rust. In order to identify the carriers of Lr68 gene, we performed PCR with the primers to STS-locus csGS-F/R. Primer sequence csGS (F: AAGATTGTTCACAGATCCATGTCA, R: GAGTATTCCGGCTCAAAAAGG), an expected amplification fragment size was 385bp (Herrera-Foessel S.A. et al., 2012). We used a Parula breed as a positive control in order to identify resistance gene Lr68. Figure 4 shows the results of electrophoresis of PCR products indicating the presence or absence of Lr68 gene in genotype of the test sample.
 |
Click here to view full figure |
M- molecular weight marker (Gene Ruler 100 bp DNA Ladder), 1-Parula, 2 – Negative control (ddH2O), 3-Almaly/GF70/1, 4-Almaly/Obryi, 5-Naz/Obriy/1MK3797, 6-Bermet/RWKLDN9, 7-BDME/Yr2, 8-Sanzar/RWKLDN9/2, 9- Almaly (225)/5347Opata85, 10- Bermet/MK3797/1, 11-Almaly (225) 5242Oxley1, 12-23/Kupava/1, 13-23/Kupava/9, 14-20/Princess/1, 15-BILINMIYEN96.7/…/ TOB // MCD/3/LIRA, 16-BEZOSTAYA1 ../ 5/F6038W12-1. 17- Avs/Naz 272.
Figure 4. DNA amplification products of wheat samples using JS2 csGS-F1/R1 primers of linked to a gene of leaf rust resistance Lr68
PCR analysis showed that in 17 lines of the studied wheat samples Lr68 gene was presented in one sample Almaly/Obryi and in positive control Parula. Evaluation of resistance to the Kazakh population of brown rust showed moderately susceptible resistance (5R-30MS).
Table 1 summarizes the results of PCR analysis using molecular markers linked to resistance genes Lr10, Lr34/Yr18, Lr37/Sr38/Yr17, Lr68 and phytopathological evaluation.
Table 1: Results of the study of promising wheat lines in the farm SP-2, Almalybak, KazNIIZR 2014
| Samples | Resistance to leaf rust in the natural conditions | Resistance to leaf rust on the background of infectious diseases | Lr10 | Lr34/Yr18 | Lr37/Sr38/Yr17 | Lr68 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Almaly/Obryi/1 | 0 | 5R | – | + | + | + |
| Almaly/Umanka/1 | 0 | 0 | – | – | – | – |
| Almaly/GF70/1 | 0 | 20S | – | – | – | – |
| Almaly/GF70/2 | 0 | 20MS | – | – | – | |
| Naz/Obri/1 | 5MS | 10MS | – | – | – | + |
| Naz/GF55/1 | 15MR | 20MS | – | – | – | + |
| Naz/GF55/4 | 0 | 10MS | – | – | – | – |
| T-425/GF55/1 | 5MR | 0 | – | – | – | + |
| T-425/GF55/2 | 10MS | 0 | – | – | – | + |
| G-428g/MK-122/2 | 15MS | 30MS | – | – | – | + |
| Bermet/RWKLDN9 | 0 | 20MS | + | – | – | – |
| Bermet/MK3797/1 | 0 | 10MR | + | – | – | – |
| BDME/Yr 2 | 0 | 5MR | – | – | – | – |
| Canzar/RWKLDN9/2 | 0 | 20MS | – | – | – | – |
| Almaly (225)/5347 Opata85/2 | 0 | 10MR | – | – | – | – |
| Almaly (225)/5347 Opata85/3 | 0 | 5MR | + | – | – | – |
| Almaly (225)/5347 Opata85/4 | 0 | 20MR | + | – | – | – |
| Almaly (225)/5242Oxley1/1 | 5MR | 0 | – | – | – | – |
| No. 23/Kupava/1 | 15MR | 30MS | – | – | – | – |
| No. 23/Kupava/19 | 0 | 5MS | – | – | – | – |
| No. 20/Knyazhna/1 | 0 | 10MR | – | – | – | – |
| TAM105/3/…/ GUN91MNCH | 0 | 20MS | – | – | – | – |
| BILINMIYEN96.7/…/ 3/LIRA | 0 | 15MS | – | – | – | – |
| BEZOSTAYA1 ..F6038W12-1 | 0 | 0 | + | – | – | – |
| Avs/Naz 272 | 0 | 20S | – | – | – | – |
| Continuation of Table 1 | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Avs/Naz 272 | 0 | 30MR | + | – | – | – |
| Avs/Naz 272 | 0 | 10MR | + | – | – | – |
| Parula 5355/293 a.2006 | 0 | 20MR | + | – | – | – |
| (Naz/Immyn78)/MK 3750 | 0 | 10MR | – | – | – | – |
| 428/Umanka | 10MR | 0 | – | – | – | + |
| 428/Umanka | 10MR | 0 | – | – | – | + |
| RWKLDN-9/Faw3750/1 | 0 | 30MS | – | – | – | + |
| Yr2/Oktyabrina | 0 | 5S | + | – | – | + |
| Almaty semi-dwarf/Progress | 10MR | 5MR | – | – | + | – |
As a result of the molecular screening of 35 promising SP2 lines studied, 20 genotypes contain Lr-resistance genes. 9 samples of wheat are carriers of the Lr10 gene: Bermet/RWKLDN9, Bermet /MK3797/1, Almaly/5347Opata85/3, Almaly/5347Opata85/4, BEZOSTAYA1/…/5/F6038W12-1, Avs/Naz 272, Avs/Naz 272, Parula 5355 /293 a.2006, Yr2/Oktyabrina. APR gene Lr34/Yr18 was found only in lines Almaly/Obriy. The complex of genes of resistance to leaf, stem and yellow rust Lr37/Sr38/Yr17 was identified only in 2 samples (Almaly/Obriy, Almaty semi-dwarf/Progress). Lr68 gene of age stability was found in 10 samples: Almaly/Obriy, Naz/Obriy, Naz/GF55, 425/GF55/1, 425/GF55/2, 428/MK-122/2, 428/Umanka, 428/Umanka, RWKLDN-9/Faw3750/1, Yr2//Oktyabrina. Genotype of the promising line Almaly/Obriy has 3 resistance genes as follows: Lr68, Lr34/Yr18, Lr37/Sr38/Yr17, while the genotype of Yr2/Oktyabrina line has 2 resistance genes: Lr10 and Lr68. Thus, these lines are the more resistant ones to rust diseases of wheat.
Conclusion
Thus, due to the possible threat of epiphytotic diseases it is necessary to create new donors of resistance to leaf rust and wheat breeding material based on them. As a result of phytopathological evaluation of susceptibility to rust on infectious background, we selected a number of samples resistant to Puccinia recondite f.sp.tritici. The use of molecular markers allowed us to study 35 samples of SP-2 wheat farm for the presence of genes of resistance to leaf rust: Lr10, Lr34/Yr18, Lr37/Sr38/Yr17 and Lr68. According to the PCR results of the relevant molecular markers linked with Lr-resistance genes, it was established that 20 wheat samples of 35 have Lr-resistance genes in the genotype. On the basis of the molecular screening of the studied wheat material, we identified nine samples with Lr10 gene, one sample with a set of genes Lr34/Yr18, 2 samples – with the complex genes Lr37/Sr38/Yr17, and 10 samples with Lr68 genes. The most valuable donor of sustainability is a promising line Almaly/Obriy, where 3 resistance genes Lr34/Yr18, Lr37/Sr38/Yr17, Lr68 and Yr2 were identified as well as Oktyabrina line with 2 resistance genes – Lr10 and Lr68. Our results provide an opportunity to move the selection process in Kazakhstan to a new scientific level through the use of molecular genetic techniques and technologies of MAS-breeding.
Acknowledgments
The authors would like to thank members of the Genetics and Selection Laboratory of the Institute of Plant Biology and Biotechnology, Department of the Gene Pool of Wildlife Plants at the Kazakh Research Institute of Agriculture and Plants for promoting the present research.
References
- Morgounov, A., 2012. Wheat exchange network breeds new life into varietal development. Date Views: 14.05.2012 http//www.cymmyt.org.
- Koishibayev, M., 2002. Diseases of cereal crops. Almaty: Bastau, pp: 368.
- Agrios, G., 2005. Plant Pathology/Fifth Edition. California: Academic Press, pp: 462.
- Bange, G.A., 2013. World wheat production. Date Views: 11.2013. http://www.usda.gov/nass.
- Tansky, V.I., M.M. Levitin, T.I. Ishkova and V.I. Kondratenko, 1998. Phytosanitary diagnostics in integrated management of cereals. Collection of guidelines on plants protection. St. Petersburg: VIZR, pp: 5-55.
- Terekhov, V.I., A.S. Kaydash and E.F. Granin 1982. Methodical instructions on the prognosis of leaf rust rate and protection of wheat crops. Moscow: Kolos, pp: 29.
- Lebedev, V.B., A.N. Vasiliev and E.V. Yakubova, 1994. Calculation of possible losses of spring wheat caused by leaf rust. Reports of VASKhNIL, 1: 14-16.
- Morgounov, A.I., H.J. Braun, H. Ketata and R. Paroda, 2006. International cooperation for winter wheat improvement in Central Asia: Results and Perspectives. Turkish Journal of Agriculture and Forestry, 29: 137-142.
- Kohmetova, A.M., Z.B. Sapahova, A.K. Madenova and G.T. Esenbekova, 2014. Identification of the carriers of genes for resistance to yellow Yr5, Yr10, Yr15 and brown rust Lr26, Lr34, based on the molecular screening of wheat samples. Biotechnology. Theory and Practice, 1: 71-78.
- McIntosh, R.A., J. Dubcovsky, J. Rogers, C. Morris, R. Appels and X. Xia, 2010. Catalogue of gene symbols for wheat: 2010 Supplement. Date Views: 03.03.2010. http://www.shigen.nig.ac.jp/wheat/komugi/genes/macgene.
- McIntosh, R.A., C.R. Wellings and R.F. Park, 1995. “Wheat Rusts: An Atlas of Resistance Genes.” Kluwer Academic Publishers, Dordrecht. DOI: 10.1007/978-94-011-0083-0
- Choudhuri, H.C., 1958. The inheritance of stem rust and leaf rust resistance in common wheat. Indian Journal of Genetics and Plant Breeding. 18: 90-115.
- Dyck, P.L., 1987. The association of a gene for leaf rust resistance with the chromosome 7D suppressor of stem rust resistance in common wheat. Genome, 29: 467-469.
- Singh, R.P. and J. Huerta-Espino, 2003. Effect of leaf rust resistance gene Lr34 on components of slow rusting at seven growth stages in wheat. Euphytica, 129: 371-376.
- Krattinger, S.G., E.S. Lagudah, W. Spielmeyer, R.P. Singh, J. Huerta-Espino, H. McFadden, E. Bossolini, L.L. Selter and B. Keller, 2009. A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science, 323: 1360-1363.
- William, H.M., D. Hoisington, R.P. Singh and D. Gonzalez de Leon, 1997. Detection of quantitative trait loci associated with leaf rust resistance in bread wheat. Genome, 40: 253-260.
- William, H.M., R.P. Singh, J. Huerta-Espino, G. Rosewarne, H.T. Buck, J.E. Nisi and N. Salomon, 2007. Characterization of genes for durable resistance to leaf rust and yellow rust in CIMMYT spring wheats. Developments in Plant Breeding. «Wheat production in stressed environments». Dordrecht. The Netherlands, 12: 65-70.
- Herrera-Foessel, S.A., R.P. Singh, J. Huerta-Espino and E.S. Lagudah, 2009. Characterization and mapping of a gene component for durable leaf rust resistance in chromosome arm 7BL. Phytopathology, 99: 53-55.
- Herrera-Foessel, S.A., R.P. Singh, J. Huerta-Espino, G.M. Rosewarne, S.K. Periyannan, L. Viccar, V. Calvo-Salazar, C. Lan and E.S. Lagudah, 2012. Lr68: a new gene conferring slow rusting resistance to leaf rust in wheat. Theoretical and Applied Genetics, 124: 1475-1486.
- Lagudah, E.S., H. McFadden, R.P. Singh, J. Huerta-Espino, H.S. Bariana and W. Spielmeyer, 2006. Molecular genetic characterization of the Lr34/Yr18 slow rusting resistance gene region in wheat. Theoretical and Applied Genetics, 114(1): 21-30.
- Singh, R.P., A. Mujeeb-Kazi and J. Huerta-Espino, 1998. A gene conferring slow-rusting resistance to leaf rust in wheat. Phytopathology, 88(9): 890-894.
- Spielmeyer, W., P.J. Sharp and E.S. Lagudah, 2003. Identification and validation of markers linked to broad-spectrum stem rust resistance gene Sr2 in wheat (Triticum aestivum L.). Crop Science, 43: 333-336.
- Dyck, P.L. and D.J. Samborski, 1979. Adult-plant leaf rust resistance in PI 250413, an introduction of common wheat. Canadian Journal of Plant Science, 59: 329-332.
- Riede, C.R. and J.A. Anderson, 1996. Linkage of RFLP markers to an aluminum tolerance gene in wheat. Crop Sci., 36: 905-909.
- Chen, X.M., R.F. Line and H. Leung, 1998. Genome scanning for resistance gene analogs in rice, barley, and wheat by high resolution electrophoresis. Theoretical and Applied Genetics, 97: 345-355.
- Timonova, E.M., I.N. Leonova, M.S. Roder and E.A. Salina, 2013. Marker-assisted development and characterization of a set of Triticumaestivum lines carrying different introgressions from the T. timopheevii genome. Molecular Breeding, 31: 123-136.
- Schachermayr, G., C. Feuillet and B. Keller, 1997. Molecular markers for the detection of the wheat leaf rust resistance gene Lr10 in diverse genetic backgrounds. Molecular Breeding, 3: 65-74.
- Helguera, M., I.A. Khan, J. Kolmer, D. Lijavetzky, L. Zhong-qi and J. Dubcovsky, 2003. PCR assays for the Lr37-Yr17-Sr38 cluster of rust resistance genes and their use to develop isogenic hard red spring wheat lines. Crop Science, 43: 1839-1847.
- Bariana, H.S. and R.A. McIntosh, 1993. Cytogenetic studies in wheat XIV. Location of rust resistance genes in VPM1 and their genetic linkage with other disease resistance genes in chromosome 2A. Genome, 36: 476-482.
- Roelfs, A.P., R.P. Singh and E.E. Saari, 1992. Rust diseases of wheat: Concepts and methods of disease management. Mexico, DF: CIMMYT, pp: 81.
- Maia, N., 1967. Obtention des bles tendres resistants au pietin-verse par croisements interspecifiques bles × Aegilops. Comptes Rendus des Seances de I ‘Academie d’Agriculture de France, 53: 149-154.

This work is licensed under a Creative Commons Attribution 4.0 International License.





