How to Cite | Publication History | PlumX Article Matrix
Reza Ranjbar1, Mehdi Moazzami Goudarzi2٭ and Nematollah Jounaidi3
1Molecular biology research center, Baqiyatallah University of medical science, Tehran, Iran.
2Department of Microbiology, Boroujerd Branch, Islamic Azad University, Boroujerd, Iran.
3Health Research Center, Baqiyatallah University of medical science, Tehran, Iran
DOI : http://dx.doi.org/10.13005/bbra/1791
ABSTRACT: The prevalence of diverse genital infections among women and their increasing resistance to antibiotics has motivated researchers to study the use of probiotics to prevent and treat them. Samples of vaginal discharge were collected from healthy married women. The types of lactobacilli present were identified by biochemical and molecular testing. The antiviral properties of a culture supernatant of wild species or strains of Lactobacillus acidophilus LA-5 on the standard strain of herpes simplex virus type 1 (HSV-1) was evaluated. L. acidophilus culture supernatant significantly decreased the amount of plaque produced by HSV- 1 on cells. Similar results were obtained for the standard strain. The inhibitory effect of the wild L. acidophilus did not require the production of H2O2 or H+ ions of the standard strain or an acidic environment. L. acidophilus was able to control HSV-1 without producing H2O2 or H+ ions or acidifying the environment. The inhibitory property of virus is most likely related to production of active metabolites.
KEYWORDS: lactobacillus acidophilus; herpes simplex virus
Download this article as:| Copy the following to cite this article: Ranjbar R, Goudarzi M. M, Jounaidi N. Lactobacillus Acidophilus and Assessment for its Antiviral Effect Against Herpes Simplex Virus Type I. Biosci Biotech Res Asia 2015;12(2) |
| Copy the following to cite this URL: Ranjbar R, Goudarzi M. M, Jounaidi N. Lactobacillus Acidophilus and Assessment for its Antiviral Effect Against Herpes Simplex Virus Type I. Biosci Biotech Res Asia 2015;12(2). Available from: https://www.biotech-asia.org/?p=2606 |
Introduction
The female reproductive system provides a balanced set of natural protective systems and the internal and external female genitalia normally protect against microbial infection and opportunistic microbes. Infectious diseases of the female genital tract occur in two forms. The first is when the natural protection provided by the genitalia disappears and the second is when a there is injury to the genital tract which makes it susceptible to infection.
The natural protective coating of the female genitalia is the most important defense. The female reproductive tract is located in close proximity to the rectum, which makes it relatively easy for colonized bacteria of the colon, especially enterobacteriaceae, to migrate to the vagina. Maintaining genito-urinary health is very important for all women, especially those with thin and sensitive skin in this area [10, 12, and 14].
Healthy female genitalia are generally colonized by lactobacilli, which create an acidic environment with a natural pH of 3.8 to 4.4. The acidic environment of the vagina stimulates and supports the growth of bacteria and inhibits the proliferation and survival of pathogens and opportunistic micro-organisms [7, 8].
Genital herpes is a sexually transmitted disease caused by the herpes simplex virus (HSV) types I and II. Infection caused by HSV-1 is seen in 15% of cases and is more common in adolescents and young adults. Exact figures are not available for Iran, but the increase in the number of referrals for patients inflicted with the condition, particularly young people, indicates increased incidence of the disease [6.10].
The products of lactobacilli, such as lactic acid, promote the growth of normal vaginal flora by inhibiting the production of H2O2 and bacteriocins from colonization by pathogens. The detection of lactobacilli in the vagina of healthy women and the ingestion or vaginal administration of products containing lactobacilli is a promising method of preventing vaginal infection that may lead to cancer of the vagina and cervix in women [4, 2].
Materials and Methods
The subjects were 100 married women 21 to 31 years of age who consulted several health centers and private clinics in Tehran over the course of three months. The subjects showed no symptoms of vaginal infection, herpes or genital warts upon examination by a physician and from the results of laboratory testing. Sterile swab samples were collected to assess the presence of lactobacilli. Each swap was cultured immediately after sampling along the MRS medium using the four-part culturing technique and was transferred to a laboratory setting for incubation.
Bacterial culture
After the transfer of the samples to the laboratory, their purity was determined by isolating the strains that were cultured on solid MRS agar under micro-aerophilic aeration conditions in Anoxomat flasks. The flasks were inverted and incubated at the 36°C. The colonies grown on the plates were opaque white, creamy, and dome-shaped. These were then subjected to isolation studies.
Fermentation of carbohydrates
An MRS agar test environment was used for isolation; the environment contained no meat extract and glucose, but contained 0.05% (w/v) red chlorophenol as an environmental marker. Phenol changes color in an acidic environment and turns the environment yellow. This test is based on the difference in the specific glucose consumption required for bacterial growth and allows determination of the different species of lactobacillus.
To test for sugar in the presence of microbes, one part per 100 ml of medium was added to the environment, which was then inoculated with a sterile loop of a microbial colony. The results were determined after 24 h of incubation. The sugars were arabinose, cellobiose, fructose, lactose, maltose, mannitol, mannose, melibiose, raffinose, rhamnose, sucrose, trehalose and xylose. Only samples displaying standard fermentation patterns for L. acidophilus were nominated for molecular identification testing. This fermentation pattern included fermented celllobiose, lactose, mannose, raffinose, rhamnose, sucrose, and xylose and did not contain fermented arabinose, melibiose and trehalose[13].
Molecular identification of L. acidophilus
PCR testing was carried out using primers specific for L. acidophilus to identify and isolate it from other strains. The primer sequences used in this test were:
- Forward: 5 ‘TGCAAAGT GGTAGCGTAAGC 3’
- Reverse: 5 ‘CCTTTCCCTCACGGTACTG 3’
These primers are universal and designed for specific acidophilus species in gene sequence 16s rRNA. The product of this PCR has a length of 170 bp and confirmed the identity of the microorganisms after sequencing. The PCR mixture was prepared using: 1 µl DNA template, 1 µl of each primer, 2.5 µl lysis buffer for PCR, 2.5 µl nucleotides, 0.2 µl taq enzyme and 16.8 µl PCR water. The final volume of the reaction mixture was 25 µl.
The PCR reaction was performed as follows: pre-denaturation at 94°C for 180 s; denaturing at 94°C for 75 s; connecting the primer at 47° for 70 s; elongation at 72°C for 60 s. This PCR cycle was repeated 24 times and final elongation was performed at 72°C for 60 s and was afterward maintained at 4°C.
Total count of living Microorganisms
The pour plate technique was used to count the probiotic bacteria. The inoculum was prepared with microorganisms grown in MRS broth (dilution was also carried out using colonies grown in MRS agar) at an apparent volume of physiologic or phosphate-buffered saline (PBS) or sterile water of specified clearance (transmittance = 0.1, λ = 625 nm).
In the first stage of dilution, 1 ml of suspension was produced using 6 sterile tubes containing 9 ml of distilled water or PBS (pH 7.4); then, 1 ml of microbial suspension or 1 of a colony grown on MRS agar was inserted into the first tube. From the first tube, 1 ml was added to the second tube and this process continued until the sixth tube was reinvigorated. The dilution of each tube differed 0.1 from the succeeding tube.
The plates from the last 3 tubes were considered separately; into each plate was poured 1 ml of each dilution and 20 ml of MRS agar was added at 45°C. The plates were rotated in a clockwise direction and the opposite until 1 ml of sample mixed with the culture medium. After sealing the environments, plates containing 5% CO2 were transferred to an incubator for 24 h, after which the number of colonies grown on the environment was counted. Plates with 30 to 300 colonies were chosen for further testing. The number of bacteria per ml was calculated as:
Number of bacteria/ml = dilution factor × average number of colonies in two plates [14].
AlsoL. acidophilus LA-5 was used as a positive control in the next series.
Preparation of supernatant
The supernatant cultures were grown on MRS broth. To prepare the supernatant for L. acidophilus, 109 colony forming units (CFU) were obtained from the total lactobacilli and transferred to 4-part plates containing 6 ml of DMEM culture medium without antibiotics and rich in glucose. The plates were incubated for 20 h in an incubator containing 5% CO2. Afterward, the plates were centrifuged at 3000 rpm at 4°C in a cold centrifuge and then filtered through a 22% µl Millipore filter. The resulting supernatant was used as the lactobacillus culture for treatment of HeLa cells before distribution of the virus.
Effect of inhibition on HSV-I
The effect of the supernatant solution on the HeLa cell strain grown in DMEM medium was evaluated to determine the antiviral properties of L. acidophilus. HSV-1 was incubated once in the absence and once in the presence of a culture supernatant (CS) of L. acidophilus. Then the effect of the supernatant of L. acidophilus culture on HeLa cell proliferation was evaluated by counting the plaque-forming units (PFU) using a plaque assay. A comparison of the total PFUs on a HeLa cell in the presence and absence of supernatant of L. acidophilus culture was the assessment criteria for effect of lactobacilli on pathogenesis of the virus.
HeLa cells were cultured to a final density of 4×105 cells per well in microplates containing DMEM medium at 37°C and in an atmosphere of 5% CO2. The microplates were examined for the formation of cell walls every 12 h under sterile conditions. When at least 98% of the cell strain had formed, it was inoculated with 50 PFUs and incubated for 1 h. The cell strain was slowly rinsed after removing the DMEM or PBS media to remove viruses that had not attached to the cells. Fresh DMEM medium was added and the contents were moved to microplates in a sterile manner and incubated for 72 h to allow infection of the cell strains. After depletion of DMEM medium, it was fixed with methanol and the platelets were stained with crystal violet and counted.
To assess the decrease in the number of platelets in the presence of lactobacilli culture, 1 mm supernatant solution containing 5%, 10%, 15% or 20% culture were prepared in sterile distilled water. The culture was added 6 h before infecting the cells with HSV-1, at the time of contamination with the virus and 6 h after contamination. The number of platelets was then counted as described. The assessments were also performed under identical circumstances with a supernatant culture of LA-5 as a standard strain.
Cytotoxicity Assay
The toxicity of the lactobacilli cells and supernatants of Vero and Hela cells was determined using the XTT proliferation assay (Roche; Germany). Vero cells were cultured in 96-well plates (6 × 103/well) (Nunc; Denmark) containing lactobacilli cells (6 × 107 CFU/ml) and CS. The bacterial cells were removed after 1 h and medium containing antibiotics was added to the wells. The medium was discarded after 72 h and the cells were then rinsed with PBS.
The effect of lactobacilli cells and supernatant on Hela cells viability was examined by subjecting HSV replication assay plates to proliferation assay directly after washing the cells with PBS. XTT reagent (40 μl/well) was added to the plates and incubated at 37°C for 3 h. The optical density was measured using an enzyme immunoassay reader (Stat Fax 2100, Awareness; USA) at test and reference wavelengths of 450 nm and 630 nm, respectively.
Results
Molecular identification
Molecular identification of bacteria was performed using specific primers after DNA purification. After 1% gel electrophoresis, PCR products with positive 170 bp bands were recorded (see figure 1). These isolates were candidates for preparation of CSs and examination of their inhibitory effect on HSV-1.
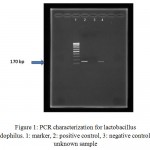 |
Figure 1: PCR characterization for lactobacillus acidophilus. 1: marker, 2: positive control, 3: negative control, 4: unknown sample |
Testing inhibition of growth of HSV-1
Figure 2 shows that the number of HSV-1 platelets produced on the HeLa cell strain declined significantly. Inhibitory effect of the supernatant solution on HSV-1 was also examined under the same conditions used for the standard strain. The results are shown in Tables 1 and 2. The cytotoxicity results showed that the CS from vaginal and non-vaginal lactobacilli was not toxic to target cells. This indicates that these strains are safe and there is selective activity of the CS in reducing of viral replication rather than cellular proliferation.
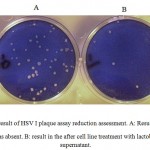 |
Figure 2: result of HSV I plaque assay reduction assessment. A: Result when the supernatant was absent. B: result in the after cell line treatment with lactobacillus culture supernatant. |
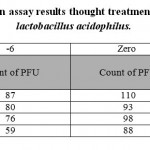 |
Table 1: plaque reduction assay results thought treatment with standard strain of lactobacillus acidophilus. |
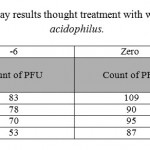 |
Table 2: plaque reduction assay results thought treatment with wild type strain of lactobacillus acidophilus. |
Discussion
Globally, different types of lactobacilli have been found to reside in the normal flora of the female genitalia. The range of lactobacilli considered to be normal microbial flora in women has changed considerably in the past 15 years [1]. A study conducted in India in 2009 reported L. fermentum, L. salvaricus and L. plantarum to be the dominant microbial flora [8, 13]. L. acidophilus has been identified as the most common species and was used in the present study to investigate the prevalence of vaginal antimicrobial effect.
It has been estimated that 50 million people in the US are infected with genital herpes. Genital herpes is spread from person to person by direct contact. It is believed that 60% of sexually-active adults carry the herpes virus. One reason for the high rate of continuous infection is that most women with high rates of infection with the virus are asymptomatic and do not know they are infected [6]. For many women, mild itching or minimal discomfort may be the only symptoms. The symptoms tend to decrease as the period of infection with HSV increases [5]. The virus can spread from the cervix into the vagina of a woman who is asymptomatic. The incidence of infection varies by country and population. The incidence of infection is 25% in American adults, 4-14% in European and Australian adults, 30-80% in African women, 10-50% in African men, and 10-30% in developing Asian countries [6,14].
The results of this study show a significant decrease in replication of HSV-1 in the presence of a supernatant culture of L. acidophilus. The remarkable virucidal capacity of lactobacilli CS is consistent with the protective effect of lactobacilli against HSV virions and suggests that the lactobacilli may secrete bacteriocins or other molecules with the capacity to neutralize the virus. This mode of anti-viral activity differs from previously reported activity because of the secretion of H2O2 and H+[2]. Because the CS used in this study had a neutral pH, it had no toxic effect on target cells. This is probably because the release of active molecules into the CS of this strain had an inhibitory effect on the proliferation of the virus.
The supernatant solution used in the present study had a neutral pH. This means that L. acidophilus did not induce its antiviral property through the production of oxygen and the release of H+ ions. The production of these two products has been formerly known as a pathway to inhibiting replication of the virus [3, 9]. The present survey also demonstrates that the maximum decrease in the cytopathic properties of HSV-1 occurred when the CS was added 6 h before infecting the cell strain with the virus. These results reinforce the hypothesis that a supernatant solution of L. acidophilusdisrupts the binding of the virus to a cell.
This mode of defense is not related to the production of H2O2 or H+ ions. The lactobacilli products may inhibit the growth of HSV by disturbing the adhesion of virions to the cells or neutralizing the viral particles. This mode has been proposed fairly recently and differs from the belief that lactobacilli inactivate viral particles by decreasing the pH and by secretion of disinfectant agents [2].
The present study shows that L. acidophilusdoes not necessarily need a cell body to inhibit virus replication; the supernatant culture of these bacteria can also inhibit the replication of HSV-1 at neutral pH in a cell culture.
Microbial vaginal flora probably inhibits the establishment and connection of HSV-1 in the early stages of infection in the host. Various mechanisms may play roles in this, including disruption of the early phases of infection formation and denaturation of the virus or the internal cells that effect reproduction of the virus in the body of the host. A comparison of the current results from wild strains in vaginal samples with standard strains of LA-5 indicate that at nearly all supernatant densities, the inhibitory power of the wild strain was greater than of the standard strain.
References
- Cherpes, T.L., Meyn, L.A., Krohn, M.A., Hillier, S.L. Risk factors for infection with herpes simplex virus type 2: role of smoking, douching, uncircumcised males, and vaginal flora.Sex Transm Dis.,2003;212(3):405–410.
- Conti, C., Malacrino, C., Mastromarino, P. Inhibition of herpes simplex virustype 2 by vaginal lactobacilli.J PhysiolPharmacol., 2009;241(1):19–26.
- Garg, K.B., I. Ganguli, R. Spectrum of Lactobacillus species present in healthy vagina of Indian women, Indian J Med Res., 2009;129: 652-657.
- McGroarty, J.A. Probiotic use of lactobacilliin the human female urogenital tract,FEMS Immunol Med Microbiol,1993; 98(5): 251-264.
- Nam, H., K. Whang and Y. Lee. Analysis of vaginal lactic acid producing bacteria in healthy women.J Microbiol, 2007;45:515-520.
- Nagot, N., Ouedraogo, A. Defer M.C., Vallo R., Mayaud P., Van P., Association between bacterial vaginosis and Herpes simplex virus type-2 infection: implications for HIV acquisition studies.Sex Transm Infect, 2007;83:365–368.
- Pabich W.L., Fihn, S.D., Stamm, W.E., Scholes, D., Boyko, E.J., Gupta, K. Prevalence and determinants of vaginal floraalterations in postmenopausal women.J Infect Dis, 2003;188:1054-1058.
- Reid, G., Burton, J. Use of Lactobacillus to prevent infection by pathogenic bacteria.Microbes Infect.,2002;231(4):319-324.
- ,grayrh., wawermj., 1infections associated with abnormal vaginal floramorphology and bacterial vaginosis. Lancet., 1997; 350(3): 530-531.
- Song, J., Abraham, S.N. Innate and adaptive immune responses in the urinary tract.Eur J Clin Invest., 2008; 38: 21-28.
- Tamrakar, R., T. Yamada, I. Furuta. Association between Lactobacillus species and bacterial vaginosis-related bacteria, and bacterial vaginosis scores in pregnant Japanese women.BMC Infect Dis.,2007;130(2):128-128.
- Vitali, B., C. Pugliese, E. Biagi., Dynamics of vaginal bacterial communities in women developing bacterial vaginosis, candidiasis, or no infection, analyzed by PCR-denaturing gradient gel electrophoresis and real-time PCR.Appl Environ Microbiol., 2007;73:5731-5741.
- Zhou, X., M.A. Hansmann, C.C. The vaginal bacterial communities of Japanese women resemble those of women in other racial groups.FEMS Immunol Med Microbiol2010;58:169-181.
- Zuckerman, R.A., Lucchetti A., Whittington, W.L., Sanchez, J., Coombs, R.W., Zuniga, R. Herpes simplex virus(HSV) suppression with valacyclovir reduces rectal and blood plasmaHIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized,double-blind, placebo-controlled crossover trial.J Infect Dis., 2007; 196:1500–1508

This work is licensed under a Creative Commons Attribution 4.0 International License.





