How to Cite | Publication History | PlumX Article Matrix
Uma Sudhakar1, T. Ramakrishnan2, A. Rekha3, H. Tamizhchelvan4, V. Shankar Ram5, Kamal kannadasan6, S. Parthiban7
1Professor and Head, Thaimoogambigai Dental college, Mugappair , Chennai -600107 2Professor, VP, Meenakshi Ammal Dental college, Mugappair , Chennai -600107 3PG student, Thaimoogambigai Dental college, Mugappair , Chennai -600107 4Professor, Oral pathology, Sri Ramachandra Dental college , Mugappair , Chennai -600107 5Reader, Thaimoogambigai Dental college, Mugappair , Chennai -600107 6Professor, Oral surgery ,Thaimoogambigai Dental college. Mugappair , Chennai -600107 7Senior lecturer - Thaimoogambigai Dental college, Mugappair , Chennai -600107
ABSTRACT: Chronic Periodontitis, an inflammatory disease caused by oral bacteria stimulates the host cells,neutrophils which release Reactive Oxygen Species(ROS) as a part of immune response. Excess ROS is one of the pathological features in the periodontal lesion. Recently, Reactive Oxygen Metabolites(ROM) were recognized as a useful measure of blood ROS .The aim of this study is to estimate the prevalence of ROM in Plasma, saliva and Gingival crevicular fluid in Chronic Periodontitis, chronic gingivitis and healthy controls. The study population consisted of 45 subjects belonging to both the sexes were randomly selected . Subjects were divided into three groups , Healthy periodontium ( HP)(Group I) and Chronic Gingivitis (CG)(Group II), Chronic Periodontitis(CP) ( Group III). GCF, saliva and plasma were collected in all the three groups to estimate the Reactive oxygen metabolite levels. ROM levels in plasma concentration were almost the same in all the groups [ pvalue 0.13]. The values obtained in saliva and GCF were significantly higher in Chronic Periodontitis group compared to Chronic Gingivitis and Healthy Periodontium.[ pvalue < 0.001]. The results of our study suggested that a significant oxidative stress may occur in Periodontitis. The findings also suggest that it might play an important role in the pathology of Periodontitis and the associated tissue damage.
KEYWORDS: ROM; plasma; saliva; GCF; Chronic Periodontitis
Download this article as:| Copy the following to cite this article: Sudhakar U, Ramakrishnan T, Rekha A, Tamizhchelvan H, Ram V. S, Kannadasan K, Parthiban S. Prevalence of Reactive Oxygen Metabolite Levels in Plasma , GCF and Saliva in Chronic Periodontitis, Chronic Gingivitis and Healthy Periodontium– A biochemical study. Biosci Biotech Res Asia 2015;12(2) |
Introduction
Periodontitis is regarded as an inflammatory lesion , mediated by complex –host Parasite interactions ,that leads to the loss of connective tissue attachment to the root surface cementum and adjacent alveolar bone 1. An emerging body of evidence is associating oxidative stress to the pathogenesis of Periodontal tissue destruction. Oxidative stress occurs when prooxidant and antioxidant balance shifts in favour of the former , leading to potential damage 2. Even though the primary etiological agent for Periodontitis is predominantly gram negative bacteria ,which initiate the tissue destruction , majority of tissue damage in Periodontitis is caused by an inappropriate host response to these microorganisms and their products3. When stimulated by pathogens , host cells(eg PMNs ) release Reactive Oxygen Species ( ROS) as a part of immune response.ROS include molecules like hydrogen peroxide, hypochlorous acid and singlet oxygen . Their excessive production by PMNs is one of the pathologic feature in periodontal lesion.ROS can cause tissue destruction by oxidizing DNA, proteins ,lipids and important enzymes such as antiproteases , stimulating proinflammatory cytokines release through depletion of intracellular thiol compounds and activating nuclear factor kappa β( NFkβ)4.
With the progression , ROS produced in the Periodontal lesion may diffuse into the blood stream and cause systemic oxidative stress. Numerous studies have suggested a positive association between Periodontitis and blood ROS levels 4. It is also known that oxidative stress makes a significant contribution to a variety of human diseases such as diabetes , arthritis , heart disease , stroke, liver disease , AIDS and Parkinsons disease5.
Reactive oxygen metabolites ( ROMs) were recognized as a usual measure of ROS .This analysis measures the level of generic peroxide present , which in turn reflects the level of free radicals from which it is formed .The test is based on the reaction of samples with transition metal ions to form alkoxyl and peroxyl radicals.
In this study , levels of ROS were investigated in plasma , saliva and Gingival crevicular fluid in Chronic Periodontitis patients . The data obtained were compared with those from healthy control and gingivitis .
Materials and Methods
Study groups
A total of 45 individuals were included in this study, 15 subjects in each group. [Chronic Periodontitis (CP),Chronic gingivitis (CG)and Health Perodontium(HP)].The subjects were chosen from out patient pool of Department of Periodontics, Thaimoogambigai Dental College and Hospital. The patients were clinically and radiographically evaluated for CP. The patients had atleast 4 teeth with one (or ) more sites exhibiting probing depth of ≥ 4 mm, Clinical attachment level (CAL) of ≥ 4 mm with radiographic evidence of bone loss . The patients with chronic gingivitis had probing depth of ≤3 mm and a CAL of ≤1 mm, with clinical signs of gingival inflammation .The control group consisted of individuals with probing depth of ≤3 mm and a CAL of ≤1 mm, with no clinical signs of gingival inflammation and who also maintained a good oral hygiene .
Among the criteria for being included in the study were patients having no systemic disease , having received no periodontal treatment , antibiotics , antinflammatory agents (or) other drugs in the last 6 months. The participants were informed about the study and their consent was obtained .The study protocol was approved by the ethical committee of Dr. M.G.R University, Chennai , India, in accordance with the Helsinki Declaration of 1975,as revised in 2000.
Collection of Samples
Collection of GCF
Detailed case history, clinical examination and supragingival scaling were done one day before the collection of GCF. On the subsequent day after drying the area with a blast of air, supragingival plaque was removed without touching the marginal gingiva and the GCF was collected. A standardized volume of 1µl was collected from each site with an extracrevicular approach , using volumetric capillary pipettes that were caliberated from 1-5µl. The collected GCF was transferred immediately to ependeroff tubes and stored at -70·c until the time of assay.
Collection of Plasma
Blood (3 ml) was drawn from the antecubital vein, under aseptic precautions and collected in a colour coded herparinised test tube (green colour) and centrifuged immediately at 3000 xg for 5 minutes. If not immediately assayed plasma aliquots were stored at -80ºc until analysis.
Collection of Saliva
Draining / Spitting method
The subject is asked to accumulate saliva in the floor of mouth and then spit into a pre-weighed or graduated test tube.
Laboratory method for detection of ROM
The d-ROMs test, developed by world-renowned Italian biochemist (Mauro Carratelli 2001)6 is a photometric test for measurement of the concentration of hydroperoxides (ROOH) in biological samples. The presence of ROOH in cells indicates oxidative attack of ROS on various organic substrates such as carbohydrates, lipids, amino acids, proteins, or nucleotides.
Test principle
The d-ROMs test uses the principle of Fenton’s reaction: by mixing a biological sample with an acidic buffer (Reagent R1), the newly-created transition metal ion (iron or copper) catalyzes the breakdown of hydroperoxide, generating new radical species such as hydroxyperoxyl (ROO+) and alkoxyl (RO+).By adding a chromogen (N, N-diethyl-paraphenylendiamine, Reagent R2) having the ability to donate an electron and change color when oxidized by free radicals, and using photometric reading available with the FRAS 4 dedicated analytical equipment, it becomes possible to quantify the level of hydroperoxides available in the sample.
Statistical Analysis
All statistical analyses were performed using a software program( Spss 15 version). Changes in the ROM levels in GCF, plasma and saliva in Health Periodontium , Chronic gingivitis and Chronic Periodontitis were analysed using one way Annova.
Results
The mean value of ROM levels in plasma , saliva and GCF in the Chronic Periodontitis , Chronic Gingivitis and Healthy Periodontium are given in Table 1.
Table 1: Comparison between groups
| N | Mean | Std. Deviation | F value | p value | |||
| GCF | HP | 15 | 274.35 | 51.84 | 71.94 | < 0.001 | |
| CG | 15 | 326.88 | 67.89 | ||||
| CP | 15 | 532.53 | 65.94 | ||||
| Plasma | HP | 15 | 287.04 | 48.14 | 2.13 | .13 | |
| CG | 15 | 375.53 | 58.32 | ||||
| CP | 15 | 577.53 | 56.88 | ||||
| Saliva | HP | 15 | 299.17 | 47.35 | 103.15 | < 0.001 | |
| CG | 15 | 377.70 | 69.44 | ||||
| CP | 15 | 592.07 | 54.44 | ||||
| *onewayANOVA *p value<0.005 | |||||||
While the ROM levels in Plasma concentration were almost the same in all groups [ pvalue 0.13] the values obtained in saliva and GCF were significantly higher in Chronic Periodontitis group compared with Chronic Gingivitis and Healthy Periodontium.[ pvalue < 0.001].
Table 2: Inferential statistics were depicted
| N | Mean | Std. Deviation | F value | p value | |||
| HP | GCF | 15 | 274.35 | 51.84 | .96 | .39 | |
| Plasma | 15 | 287.04 | 48.14 | ||||
| Saliva | 15 | 299.17 | 47.35 | ||||
| CG | GCF | 15 | 326.88 | 67.89 | 1.27 | .29 | |
| Plasma | 15 | 570.97 | 58.32 | ||||
| Saliva | 15 | 377.70 | 69.44 | ||||
| CP | GCF | 15 | 532.53 | 65.94 | 4.11 | .02 | |
| Plasma | 15 | 577.53 | 56.88 | ||||
| Saliva | 15 | 592.07 | 54.44 | ||||
*p value<0.005-significant.
There was no significant difference in ROM values in GCF, plasma and saliva in Healthy Periodontium and chronic gingivitis [p value 0.39,0.29]respectively. However there was a significant difference in ROM levels in Chronic Periodontitis.
[p value .02].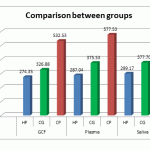 |
Figure 1: Comparison between groups |
Discussion
To the best of our knowledge , this is one of the few studies performed to estimate the level of ROM in Plasma ,Saliva and GCF in Chronic Periodontitis , Chronic Gingivitis and Healthy Periodontium. Several approaches are possible to demonstrate the involvement of oxidative stress in the pathophysiologic mechanisms of diseases. One of these approaches in the assay of end products of peroxides.[Romeno etal 1998]7.
In the present study , while the plasma ROM levels in the Chronic Periodontitis did not differ significantly compared with Chronic Gingivitis and Healthy Periodontium . Saliva and GCF values showed significant increase and the highest value was observed in GCF. Our results are in accordance with the studies done by [ Sobanice .H and Sobanice Lotowska 20008, Sheikhi etal 20019,Tuter et al 200110, Panjamoorthy etal 200511, Tsai etal 2005 12].
A possible association of significantly higher LPO concentrations with an increased percentage of GCF in Periodontitis patients was reported (Tsai etal 2005)12. Increased GCF flow relates to increasd PMN levels , which inturn contributes to over all peroxidise enhancement by myeloperoxidase activity ( Battino etal 2002)14. Moreover the increase level of ROM in saliva , in Chronic Periodontitis in the present study could be partly due to an increased action of ROS in the saliva itself against the increasing amount of bacteria and their products and partly due to an increased leakage of ROS to saliva from plasma and GCF.
Disturbances of balance between ROS and antioxidants contributes significantly to the development of inflammatory oral diseases( Chapple 199713, Battino etal 20020)14.
ROM levels in GCF, which were higher than those in plasma and saliva in the present study showed that a local increase in ROS level is more prominent in the Periodontal pocket in Chronic Periodontitis and was more significant than the systemic increase in terms of periodontal disease pathology . This is similar to the study by Akalin etal 20074.
While unstimulated saliva was collected in this study (Swalleyand Langly Evans 2003)15as it represents the major intraoral condition regarding the saliva rate and composition . It also contains some elements of GCF and tissue metabolites that may be useful in the determination of tissue degradation (Kaufman and Lamster 2000)16. In addition ,stimulating Saliva flow has been demonstrated to increase saliva volume and disrupt the concentration( Moore etal 1994)17.
Conclusion
In conclusion , the results of our study suggest that a significant oxidative stress may occur in Periodontitis. The findings also suggest that it might play an important role in the pathology of Periodontitis and the associated tissue damage. Oxidative stress plays an important role in the pathologies related to smoking and diabetes which are among the risk factors of Periodontitis. [Loe 199317, Toretti and Claffey 200519].
Further longitudinal studies in a larger sample size could be helpful in depiciting the role of ROS metabolites in periodontal health , which would be beneficial in treating the multifactorial disease.
References
- Page RC, Kornman KS. The Pathogenesis of Human Periodontitis .An introduction . Periodontol 1997;147-11.
- Sics H. Oxidative stress: oxidants& antioxidants. New York academic Press 1991.
- Lamster IB and Novak MJ. Host mediators in GCF. Implications for the pathogenesis of periodontal disease. Critical Rev Oral Bio Med 1992;3:31- 60.
- Akalins FA, Baltacoglu E, Alver A. Lipid Peroxidation levels and total oxidant status in serum , saliva and GCF in Patients with Chronic Periodontitis . Journal of Clinical Periodontology 2007,34:558-65.
- Mc Cord J.M. The evolution of free radicals and oxidative stress. Am J. Med 2000; 108;652-9.
- Mauro Carratelli. Caratellis Panel for global assessement of oxidative stress. Italian Journal of Medicine , 2001.
- F.J, Bosch.Morell F, Romeno M.J, Fameno E.J, Romeno.B. Lipid peroxidation products and antioxidants in human disease. Environmental Health Perspective 1998; 106( supplement 5)1229-1234.
- Sobanice .H and Sobanice –Lotowska , M.E.Morphological examinations of hard tissue of periodontium and evaluation of selected process of lipid peroxidation in blood serum of rats in the course of experimental periodontitis. Medical Science Monitor 2000; 6, 875-881.
- Sheikhi .M, Bouhaf R.K , Hammarstrom. K.J and Jarstrand .C. Lipid peroxidation caused by oxygen radicals from fusobacterium –stimulated neutrophils as a possible model for emergence of periodontitis.Oral Diseases 2001: 7, 41-46.
- Tuter .G, Kurthe B and Sudar .M. Interleukin-1 beta and thiobarbituric acid reactive substance (TBARS) levels after phase I periodontal therapy in pateints with Chronic Periodontiitis. Journal of Periodontology 2001;72, 883-888.
- Panjamurthy .K, Manoharan .S and Ramachandran .C.R. Lipid peroxidation and antioxidant status in pateints with Periodontitis. Cellular and Molecular Biology Letters 2005:10,225-264.
- C.C,Chen H.S, Chen S.L, HO.YP, WU Y.M and Hung.C.C.Lipid peroxidation –a possible role in the induction and progression of Chronic Periodontitis. Journal of Periodontology 2005;72,883-888.
- I, L.C, Mason. I, Garner I, Mathews J.B, Thorpe.G.H, Maxwell.S.R.J and Whitehead .T.P.Enhanced chemiluminescent assay for measuring the total antioxidant capacity of serum , saliva and GCF. Annals clinical Biochemistry 1997;34,412-421.
- Battino .M, Bullon.P, Wilson.M.E and Newman.H. The antioxidant capacity of saliva. Journal of Clinical Periodontology 2002;29,189-194.
- Sculley D.V, and Langley .Evans.S.C. Periodontal disease is associated with lower antioxidant capacity in whole saliva and evidence of increased protein oxidation. Clinical science 2003;105, 167-172.
- E and Lamster I.B. Analysis of saliva for Periodontal diagnosis . a review.Journal of Clinical Periodontology .2000;27, 453-465.
- S, Calder K.A, Miller N.J and Rice Evans .C.Antioxidant activity of saliva and Periodontal disease. Free radical research. 1994; 21,417-425.
- H.Periodontal disease . The sixth Complication of Diabetes Mellitus. Diabetes care 1993;16, 329-324.
- Toretti ,M.S and Claffey .N. Group C consensus report of the 5th European workshop in Periodontology.Advances in the progression of a Periodontitis case and disease progression for use in risk factor research. Journal of Clinical Periodontology 2005;32( supplement 6),210-213.

This work is licensed under a Creative Commons Attribution 4.0 International License.





