How to Cite | Publication History | PlumX Article Matrix
Preethi Murugan, Preethi Jampala, Swathy Ramanujam and Kiran Babu Uppuluri*
Bioprospecting Laboratory, Department of Biotechnology, School of Chemical and Biotechnology, SASTRA University, Thanjavur-613401, Tamil Nadu, India
ABSTRACT: Xylanase (endo-1, 4-β-xylanase) is an industrially important enzyme that hydrolyses hemicellulose, xylan into xyloligosaccharides, xylotriose, xylobiose and xylose. Commonly xylanase can be produced by a wide variety of microorganisms. It is widely used for hemicellulosic hydrolysis especially in biofuel production, textile and paper manufacture. The present work aims the production of xylanase by co-culturing Acetobacter xylinum NCIM 2526 and Cellulomonas uda NCIM 2353 utilizing a novel and cheap lignocellulosic substrate, Prosopis juliflora under submerged fermentation (SmF). This traditional approach using a mixed microbial culture and cheap substrates can reduce the enzyme production cost. Growth patterns and xylanase production profiles of chosen bacteria were studied in both mono and mixed cultures. Mixed culture of Acetobacter xylinum and Cellulomonas uda was produced higher xylanase titers than the mono-cultures. Maximum xylanase activity, 5.85 U/ml was obtained using 3% pods in 72 hours of fermentation with the mixed microbial culture. This may be due to efficient use of substrates as compared to their respective mono-cultures.
KEYWORDS: Acetobacter xylinum; Cellulomonas uda; Prosopis juliflora; Xylanase; Mixed culture
Download this article as:| Copy the following to cite this article: Murugan P, Jampala P, Ramanujam S, Uppuluri K. B. Production of Xylanase from A Mixed Culture System of Acetobacter Xylinum and Cellulomonas Uda in Submerged Fermentation. Biosci Biotech Res Asia 2015;12(2) |
Introduction
The use of lignocellulosic biomass as a raw material has gained much attention for the production of enzymes, secondary metabolites and biofuels. Plant biomass is considered as a waste material and is an important source of lignocellulosic biomass [1]. They are usually left to rot or burned in the field after harvesting and causing environmental pollution. Most of these agro-industrial residues in million tones are wasted every year; hence these can be utilized as a major substrate for the industrial fermentation process. One of the potential applications could be using these low cost lignocellulosic substrates for the production of industrially important enzymes, as they offer a good cellulosic source [1, 2].
Xylanase is an industrially important enzyme finds a wide variety of applications. It is used for bioconversion of lignocellulosic substrates into sugar [3, 4], clarification of fruit juices and wine [5], in paper industries for pulp treatment [2], textile industry and so forth. Lignocelluloses waste consists of cellulose and hemicellulose requires synergistic action of enzymes to breakdown polysaccharides into simple sugars [6]. For converting hemicellulose into monomeric sugars (xylose) many xylanolytic enzymes are involved; endo 1,4- β- xylanases (EC 3.2.1.8), β-d-xylosidases (EC 3.2.1.37), α-l-arabinofuranosidases (EC 3.2.1.55), α-D-glucuronidases (EC 3.2.1.139) and acetylxylanesterases (EC 3.1.1.72) [7]. Bacteria, filamentous fungi, actinomycetes and yeast were found to produce xylanolytic enzymes [1, 8]. The production is regulated by physiological and biochemical nature of the microbes. Synergistic action of enzymes can be improved by the use of mixed culture or co-culture.
Mixed culture could be developed by simultaneously culturing of two or more different species or the species of same kind in the fermentation media; hence inoculum of a medium contains two or more microorganisms. This type of fermentation helps in the enhanced production of antibiotics, enzymes, dairy fermentation and wastewater sludge, amino acids, vitamins [6, 9, 10]. It was reported that co-culture of Aspergillus niger and T.reesei enhanced the production of xylanase under submerged fermentation. They also claimed that this could be due the synergistic effect of both the enzymes [11]. Synergistic effect of enzymes helps in easy degradation of heteropolymers and efficient substrate utilization. Some of the advantages of mixed microbial cultures are nutritional limitation [12], easy adaptable to the changing environmental conditions and increased resistance to contamination by unwanted microbes. Mixed cultures follows a continuous and cooperative reaction and the product of one enzyme reaction becomes the substrate for the other enzymes [9, 13] and reduces overall cost of production. They also offer better saccharification of substrates and efficient substrate utilization, increases biomass and enzyme production than mono-cultures in addition to its less fermentation process time [9]. So far many fungi were co-cultured, most commonly Trichoderma reesei and Aspergillus niger, for the production of xylanase on various substrates. This is because, combination of these two resulted in substantial amount of xylanase. Some of examples of mixed cultures used for the production of xylanase are listed in the Table 1. Hence mixed microbial culture production was studied from past two decades for enzymes, antibiotics, amino acids and organic acids. Especially enzymes like amylase, xylanase, cellulases, β-glucosidase and protease were reported previously [14].
Table 1: Mixed microbial cultures used for the production of xylanase
| S.NO | Mixed culture | Substrate | Yield | References |
| 1 |
Trichoderma reesei cocultured with Aspergillus niger
|
sugar cane bagasse | 2,600–2,800 IU/g dry wt | [28] |
| 2 | Penicillium oxalicum and Pleurotus ostreatus
|
sugarcane bagasse and black gram husk | 8205.31 IU/g
|
[6] |
| 3. | T. reesei and Aspergillus niger
|
Rice chaff | 5.64 IU/gds | [16] |
| 4 | Trichoderma reesei and A. phoenicis | Sugarcane baggase | 5,500–5,900 IU/L.h. | [28] |
| 5 | Aspergillus niger MSK-7 and
Trichoderma viride MSK-10 |
Wheat bran | 189.7 U/ml/min | [29] |
| 6 | Trichoderma reesei and Aspergillus niger
|
Water hyacinth | 21. 4 g/l | [11] |
| 7 | Trichoderma reesei and Aspergillus oryzae
|
Wheat bran | 10.7 IU/gds | [30] |
| 8 | Aspergillus flavus and Cladosporium sphaerospermum and Epicoccum purpurascens | Wheat bran, corn cobs and saw dust | 55.41 U/g
|
[5] |
| 9 | Bacillus polymyxa and Cellulomonas uda | grass extract and sugarcane sheath leaf | 14.662 IU/ml | [31] |
| 10 | A.niger and Fusarium oxysporum | Forest waste | 669.08 U/g | [32] |
Presently the major bottleneck for industries producing enzymes is high cost of enzyme production since a high cost of carbon source is required as a fermentative substrate. So in order to overcome such situations, mixed cultures and lingocellulosic substrates rich in carbon source are used [15]. So far, wheat bran [16], sugarcane baggase [17], rice straw [18], wheat straw [19], corn steep liquor, lactose [19], apple pomace [20], tomato pomace and agro-industrial by-products are used for xylanase production. In the present srudy, Prosopis juliflora a novel and cheap lignocellulosic substrate is used. P.juliflora is a woody biomass which is adapted to the drylands [21]. These are not utilized by animals as a fodder because of its neurotoxin effects and indigestivity of pods [22]. The dried pods of P.juliflora have been characterized and it contains cellulose, lignin, hemicellulose. Being low cost substrates, this can serve as inexpensive fermentation substrates.
The objective of the present research work is to evaluate the biosynthesis of xylanase by mixed microbial culturing of Acetobacter xylinum NCIM 2526 and Cellulomonas uda NCIM 2353 in batch experiments under submerged fermentation. P.juliflora was used as the substrate. The production of xylanase was compared with the xylanase produced in mono-cultures of A. xylinum and C.uda.
Materials and Methods
Microorganisms and Maintenance
Acetobacter xylinum NCIM 2526 and Cellulomonas uda NCIM 2523 was procured from National Chemical laboratory, Pune, India. The obtained cultures were maintained in nutrient agar plates. The growth media for Acetobacter xylinum consists of (g L-1 ) peptone, 5.0; yeast extract, 1.5; Beef extract, 1.5; NaCl, 5; having a pH of 6.8. Cellulomonas uda was maintained in Dubos medium. The medium consisted of (g L-1): NaNO3, 0.5; KH2PO4, 1.0; MgSO4.7H2O, 0.5; KCl, 0.5; FeSO4.7H2O, 0.01; yeast extract, 0.5; Carboxymethylcellulose (CMC), 5.0; maintained at a pH of 6.5. The culture was sub-cultured for every one month interval and stored at 4 ̊C. All the chemicals were procured from Merck, HiMedia and Sigma.
Pods Collection
Prosopis juliflora pods were collected from in and around SASTRA University campus, Thanjavur, Tamil Nadu, India. The collected pods were dried and grounded to course powder using mixer grinder. To get a uniform size, the pods were passed through the sieve #40 and retained on sieve #60 and dried in the oven for (50 ̊C). The substrates were collected from same region to avoid bias due to change in nutritional content.
Growth pattern of A.xylinum and C.uda in mono culture system using P. juliflora
Both microorganisms were individually inoculated in the pods containing media to study the support of P. juliflora as a major substrate as well as the growth pattern of individual microorganisms. The medium used for xylanase consists of (g L-1): pods, 5; glucose, 5; KH2PO4, 10; (NH4)2SO4, 7; MgSO4.7H2O, 0.3; CaCl2, 1.5; urea, 1.5; peptone, 0.1; tween80, 0.1ml [23, 24]. The production media was autoclaved for 20 min at 15Lb/in2. The autoclaved media was inoculated with 5% inoculum and kept in orbital rotary shaker for 5 days. The samples were collected for every 6h intervals and the absorbance of the culture was read at 600nm.
Growth pattern of A.xylinum and C.uda in mixed microbial culture system using P. juliflora
A.xylinum and C.uda at 5% v/v was co-cultured in above mentioned sterile medium. The culture was incubated for 5 days in orbital rotary shaker at 30oC. To investigate the growth pattern, culture was sampled periodically for every 6h and its Optical Density (OD600nm) was determined using spectrophotometer.
The dry weight was determined by measuring the solid dry weight of the cells. The co-cultured samples were collected for every 6h interval for biomass determination. The dry weight was determined by centrifuging the culture (10000rpm for 10 min). The obtained supernatant was discarded and the pellet was dried in hot air oven (50 ̊C) to determine its dry weight.
Xylanase production by C.uda and A.xylinum in mono culture systems using P. juliflora
Different concentrations of pods (1-6%) were added in production media containing above mentioned constituents and the pH was adjusted to 7.4. The media was autoclaved and inoculated with 5% of A.xylinum and C.uda and incubated at 30 ̊C under shaking for 4 days.
Samples were collected for every 4h intervals and centrifuged at 10000 rpm for 10 min at 4 ̊C. The clear cell-free supernatant was assayed for xylanase activity.
Xylanase Production by C.uda and A.xylinum in Mixed Culture Systems Using P. juliflora
The production media was prepared containing pods (1-6%) in 250 ml Erlenmeyer flask. The sterilized media was co-cultivated with 5% A.xylinum and C.uda simultaneously and incubated at 30 ̊C under shaking for 5 days. After incubation, centrifugation was done at 10000 rpm for 10 min at 4 ̊C. The resultant supernatant was used to estimate the xylanase activity.
Xylanase Assay
Xylanase activity was determined by dinitrosalicylic acid method (DNS) which measures the reducing sugars, xylose from Beech wood xylan (1%, w/v). The reaction mixture containing 0.5ml of xylan and 0.5ml of crude enzyme in citrate phosphate buffer, pH 5.4 was incubated for 30min at 37 ̊C [23]. One unit of xylanase was defined as the amount of enzyme required to release 1µmol of xylose from xylan in 1 min under the assay condition.
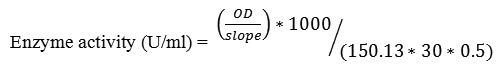
Where:
150.13 = molecular weight of xylose (g/mol)
30= reaction time (min)
0.5= volume of enzyme taken (ml)
Results and Discussion
Growth pattern of A.xylinum in mono culture system using P. juliflora
Bacterial growth phase can be identified by determining the biomass of cells or by measuring the optical density (OD600nm). In this study, the growth pattern of the A.xylinum was observed to determine its optical density. All the phases (lag, log, stationary and decline) of the bacteria in batch culture system were observed (Fig. 1). Initially in the lag phase (0-6th h), there was no indication of growth but traces of biomass was detected. This is due to the reason that the microorganisms didn’t utilize much of the substrate initially. Later, A.xylinum showed a rapid exponential phase from the 10h (OD: 0.17) to till 36h (OD: 0.94) and reached its maximum growth at 48h. This is may be due to the efficient hydrolysis of the substrate by formed enzymes during lag phase and multiplication of the microbes started which indicated the exponential growth. The growth was indicated by turbidity in the media. The growth increased slowly before reaching the maximum OD (1.167) at 48th h. 50-64th h was marked as stationary phase which resulted due to the growth-limiting factors such as depletion of essential nutrients, formation of inhibitory products [25]. Finally, due to lack of nutrients and production of more inhibitory substances the cells begin to die. This suggested that the log phase could support for optimum growth in the production media.
 |
Figure 1: Growth profile of A.xylinum and C.uda with respect to time in LB broth |
Growth pattern of C.uda in mono culture system using P. juliflora
Similarly the optimum growth of the C.uda was determined by measuring its optical density for every 6h. The growth characteristics with different phases were identified for estimating the maximum time for its growth (Fig. 1). At 0- 10h, lag phase was observed were the cells initially adapted themselves to the growth conditions. The growth curve was almost similar to A.xylinum. The growth rate increased slowly and reached a maximum at 54h. The rate of multiplication of cells increased rapidly which indicated the growth. This showed that C.uda utilized the pods efficiently and adapted well in the media. Thus the time required to reach the maximum growth is 54h for C.uda.
Growth pattern of A.xylinum and C.uda in mixed culture system using P. juliflora
Mixed microbial (A. xylinum and C.uda) growth and production profiling in pods containing medium was also established. Growth pattern of the mixed culture in terms of both optical density and biomass was shown in Fig. 2. The growth curve for the mixed culture was almost similar to the mono-culture. The maximum growth was found at the late exponential phase, 54h. After sometime the growth dropped drastically to an optical density of 1.13.
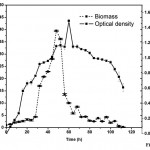 |
Figure 2: Growth pattern (Biomass Vs Optical Density Vs Time) for mixed microbial (A. xylinum & C. uda). |
The dry biomass production in mixed culture resulted in an increase in fermentation parameters like volumetric increase in xylanase production and higher concentration of reducing sugars. Initially the biomass production was low but after 20h the production increased slowly. The productivity reached its peak at 52h with 39.5g/l. This is the higher productivity may be due to the action of microbial partners in the media.
Xylanase production by C.uda and A. xylinum in mono culture system using P. juliflora
To study the xylanase production profiling in mono culture system, xylanase activity was determined for the samples collected for every 4h from different media containing concentration of pods i.e. 1-5%. The maximum enzyme activity was obtained at 48h for A. xylinum and 54h for C.uda.
The maximum xylanase activity for 1%, 2%, 3%, 4%, 5% and 6% pods is 0.34 U/ml, 0.44 U/ml, 0.58 U/ml, 0.70 U/ml, 0.84 U/ml, and 0.72 U/ml respectively for C.uda. The xylanase production profile for 1% and 2% are almost similar where the production started after 12 hours of fermentation and increased gradually. It reached the maximum at 54h (Fig. 3). In 3% pods, production dropped at 50h but again production peaked at 54h. When compared to 4%, 5% and 6%, the xylanase activity was low in 6% pods. This may be due the reason that higher concentration of pods had a negative effect on xylanase production. Thus, by comparing the time profile for all the pod concentration, 54h was considered to be the optimum time for the maximum xylanase production in 5% pods containing medium.
 |
Figure 3: Xylanase production profile by C. uda in a media containing different concentrations of Prosopis juliflora. |
For A.xylinum, maximum xylanase activity for 1%, 2%, 3%, 4%, 5% are 0.89 U/ml, 1.074 U/ml, 1.26 U/ml, 0.68 U/ml, and 0.55 U/ml respectively. After 50h, there was a sudden decrease in the growth and production for all the pod concentration (Fig. 4). This is because of the depletion of nutrients, sudden change of environmental conditions [22]. It was observed that the concentration of reducing sugar released was closely related to the amount of xylanase produced [14, 26]. 3% pods gave the maximum xylanase (1.26 U/ml) at 48h. Thus, 48h and 3% pods were found to be optimum for maximum xylanase production and were used for the analysis of xylanase in mixed culture.
 |
Figure 4: Xylanase production profile by A. xylinum in a media containing different concentrations of Prosopis juliflora. |
Xylanase production by C.uda and A.xylinum in mixed culture system using P. juliflora
The mixed culture containing 3% pods produced a tremendous increase in xylanase activity when compared to mono-cultures (Fig. 5). The mixed enzyme preparation of xylanase was developed by co-cultivating A.xylinum and C.uda. The maximum xylanase activity was observed to be 5.85 U/ml which was 4 times higher than the mono-culture of A.xylinum and C.uda – 1.26 U/ml and 0.84 U/ml respectively (Fig. 6).
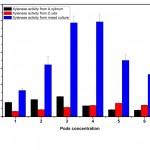 |
Figure 5: Xylanase production at different concentrations of Prosopis juliflora by A. xylinum, C. uda and mixed culture. |
 |
Figure 6: Xylanase production profile by mixed microbial culture in 3% P.julifora containing medium |
It has been established that strain compatibility and nutritional availability to the microbes are vital for better mixed culture fermentation [14]. A.xylinum and C.uda grew well in media containing pods and increased activity was observed in the mixed culture. This is due to the synergistic effect of cellulolytic and xylanolyitc enzymes on substrate under the influence of submerged fermentation which lead to the greater efficiency of substrate degradation [6]. Since the growth profile for the A.xylinum and C.uda was almost similar, they were co-cultured to regulate the complete utilization of medium components. High concentration of reducing sugars was produced in mixed culture indicating higher xylanase production. The converted reducing sugars can be further fermented to valuable products and used for the production of bio-fuels, antibiotics [14].
The use of mixed microbial culture produces higher enzyme production due to their synergistic action of enzymes [27]. Similarly in this present study, mixed culture showed a tremendous increase in xylanase activity (from 1.26 to 5.85 U/ml). The substrate, Prosopis juliflora utilization was efficient and the comparison of xylanase production was compared with the existing literature.
So far from the literature survey it was found that fungi have been used as a co-culture medium for enzyme production. The microbial partners Trichoderma reesi and Aspergillus sp. has been used widely for the xylanase production on different substrate as listed in the table 1. This is because, combination of these two resulted in substantial amount of xylanase and suggested that the sequential action of two benefited three enzyme components in the complex [14]. The maximum activity produced was 2,600–2,800 IU/g on sugarcane baggase [28] which was higher than the mono-culture (T.reesei and Aspergillus sp.). Although, when this combination grew on rice chaff, the production was 5.64 U/gds which was almost equal to the xylanase produced by Acetobacter xylinum and Cellulomonas uda which indicated the efficient utilization of the substrate P.juliflora. This was due to the reason that the mixture increased the degree of saccharification [20]. Thus, the use of co-cultures when grown on hemicellulose rich sources can be an efficient way to increase the enzyme production.
The use of bacteria in mixed culture has gained attention for enzyme production because they secrete high levels of enzymes in the medium. The use of mixed bacterial species for xylanase production is less, so the combination A.xylinum and C.uda can be used by growing them on P.juliflora substrate. Being rich in carbon source it can be used for xylanase production and thus lowering the production cost.
Conclusion
The results indicate that mixed culture of A.xylinum and C.uda can be used for enhancing the xylanase production when compared to mono-culture. The cellulose and hemicellulose degradation was significantly higher than that of mono culture which resulted in higher concentration of reducing sugars. Since the symbiotic association of these two strains in mixed culture led to efficient degradation, high concentration of reducing sugars was produced. Use of this low cost substrate resulted in low fermentation process and better xylanase activity. To favor the present problem faced by the industries due to the high cost of carbon sources, this mixed submerged cultivation can be used for the production of various targeted applications. Further investigation will be optimization of medium components by statistical methods so that this mixed culture system can be adopted in industries for better enzyme production. Good levels of carbon source, readily, abundantly and cheaply availability of P. juliflora makes the substrate more suitable for the fermentation industry and can be an important one in future for other metabolites and bio-fuel production.
Acknowledgements
This work was supported by the grant (SB/FTP/ETA-212/2012) by SERB, Department of science and technology, India.
References
- Bajaj, B.K., Y.P. Khajuria, and V.P. Singh, Agricultural residues as potential substrates for production of xylanase from alkali-thermotolerant bacterial isolate. Biocatalysis and Agricultural Biotechnology, 2012. 1(4): p. 314-320.
- Paulová, L., et al., Lignocellulosic ethanol: Technology design and its impact on process efficiency. Biotechnology advances, 2014.
- Bocchini, D., et al., Optimization of xylanase production by Bacillus circulans D1 in submerged fermentation using response surface methodology. Process Biochemistry, 2002. 38(5): p. 727-731.
- Saha, S.P. and S. Ghosh, Optimization of xylanase production by Penicillium citrinum xym2 and application in saccharification of agro-residues. Biocatalysis and Agricultural Biotechnology, 2014. 3(4): p. 188-196.
- Mostafa, F.A., A.A.A. El Aty, and H.R. Wehaidy, Improved Xylanase production by mixing low cost wastes and novel co-culture of three marine-derived fungi in solid state fermentation. Int. J. Curr. Microbiol. App. Sci, 2014. 3(7): p. 336-349.
- Dwivedi, P., et al., Co-cultivation of mutant Penicillium oxalicum SAU E-3.510 and Pleurotus ostreatus for simultaneous biosynthesis of xylanase and laccase under solid-state fermentation. New biotechnology, 2011. 28(6): p. 616-626.
- Motta, F., C. Andrade, and M. Santana, A review of xylanase production by the fermentation of xylan: Classification, characterization and applications. Sustainable Degradation of Lignocellulosic Biomass-Techniques, Application and Commercialization, 2013: p. 251-275.
- Ariffin, H., et al., Production and characterization of cellulase by Bacillus pumilus EB3. International Journal of Engineering and Technology, 2006. 3(1): p. 47-53.
- Tesfaw, A. and F. Assefa, Co-culture: A Great Promising Method in Single Cell Protein Production. Biotech. & Mol. Biol. Review. 9 (2): 12, 2014. 20.
- Alam, M.Z., et al., Optimization of compatible mixed cultures for liquid state bioconversion of municipal wastewater sludge. Water, Air, and Soil Pollution, 2003. 149(1-4): p. 113-126.
- Deshpande, S., et al., Production of cellulase and xylanase by Trichoderma reesei (QM 9414 mutant), Aspergillus niger and mixed culture by solid state fermentation (SSF) of water hyacinth (Eichhornia crassipes). Indian Journal of Chemical Technology, 2008. 15(5): p. 449.
- Castillo, M.R., et al., Mixed culture solid substrate fermentation for cellulolytic enzyme production. Biotechnology Letters, 1994. 16(9): p. 967-972.
- Ryu, D.D. and M. Mandels, Cellulases: biosynthesis and applications. Enzyme and microbial technology, 1980. 2(2): p. 91-102.
- Ahamed, A. and P. Vermette, Enhanced enzyme production from mixed cultures of Trichoderma reesei RUT-C30 and Aspergillus niger LMA grown as fed batch in a stirred tank bioreactor. Biochemical Engineering Journal, 2008. 42(1): p. 41-46.
- Salehizadeh, H. and M. Van Loosdrecht, Production of polyhydroxyalkanoates by mixed culture: recent trends and biotechnological importance. Biotechnology advances, 2004. 22(3): p. 261-279.
- Singhania, R.R., et al., An integrative process for bio-ethanol production employing SSF produced cellulase without extraction. Biochemical Engineering Journal, 2015.
- Ladeira, S.A., et al., Cellulase production by thermophilic Bacillus sp. SMIA-2 and its detergent compatibility. Electronic Journal of Biotechnology, 2015.
- Kaur, B., H. Oberoi, and B. Chadha, Enhanced cellulase producing mutants developed from heterokaryotic Aspergillus strain. Bioresource Technology, 2014. 156: p. 100-107.
- Sukumaran, R.K., R.R. Singhania, and A. Pandey, Microbial cellulases-production, applications and challenges. Journal of Scientific and Industrial Research, 2005. 64(11): p. 832.
- Dhillon, G.S., et al., Improved xylanase production using apple pomace waste by Aspergillus niger in Koji fermentation. Engineering in Life Sciences, 2012. 12(2): p. 198-208.
- Shitanda, D., et al., Properties of Prosopis juliflora and its potential uses in ASAL areas of Kenya. Journal of Agriculture, Science and Technology, 2014. 15(1).
- Ramasamy, S., et al., Production and Statistical Optimization of Oxytetracycline from Streptomyces rimosus NCIM 2213 using a New Cellulosic Substrate, Prosopis juliflora. BioResources, 2014. 9(4): p. 7209-7221.
- Li, Y., et al., Statistical optimization of xylanase production from new isolated Penicillium oxalicum ZH-30 in submerged fermentation. Biochemical Engineering Journal, 2007. 34(1): p. 82-86.
- Ang, S., et al., Production of cellulases and xylanase by Aspergillus fumigatus SK1 using untreated oil palm trunk through solid state fermentation. Process Biochemistry, 2013. 48(9): p. 1293-1302.
- Rathnan, R.K. and T. Balasaravanan, Comparative study of saccharification of fruit waste by mono and mixed cultures of cellulolytic fungi. Int. J. Curr. Microbiol. App. Sci, 2014. 3(6): p. 958-970.
- Kondo, T. and M. Kondo, Efficient production of acetic acid from glucose in a mixed culture of Zymomonas mobilis and Acetobacter sp. Journal of fermentation and bioengineering, 1996. 81(1): p. 42-46.
- Wen, Z., W. Liao, and S. Chen, Production of cellulase/β-glucosidase by the mixed fungi culture Trichoderma reesei and Aspergillus phoenicis on dairy manure. Process Biochemistry, 2005. 40(9): p. 3087-3094.
- Gutierrez-Correa, M. and R.P. Tengerdy, Xylanase production by fungal mixed culture solid substrate fermentation on sugar cane bagasse. Biotechnology Letters, 1998. 20(1): p. 45-47.
- Ikram-ul-Haq, M.M.J. and T.S. Khan, An innovative approach for hyperproduction of cellulolytic and hemicellulolytic enzymes by consortium of Aspergillus niger MSK-7 and Trichoderma viride MSK-10. African Journal of Biotechnology, 2006. 5(8): p. 609-614.
- Brijwani, K., H.S. Oberoi, and P.V. Vadlani, Production of a cellulolytic enzyme system in mixed-culture solid-state fermentation of soybean hulls supplemented with wheat bran. Process Biochemistry, 2010. 45(1): p. 120-128.
- Moses Jeyakumar, R. and L. Rajesh, Effect of various physical parameters for the Production of the enzyme Xylanase from mixed culture of Bacillus polymyxa and Cellulomonas uda. Asian Journal of Biomedical and Pharmaceutical Sciences, 2012. 2(14): p. 72-74.
- Kaushal, R., N. Sharma, and D. Tandon, Cellulase and xylanase production by co-culture of Aspergillus niger and Fusarium oxysporum utilizing forest waste. Turk J Biochem, 2012. 37: p. 35-41.

This work is licensed under a Creative Commons Attribution 4.0 International License.





