How to Cite | Publication History | PlumX Article Matrix
Uma Sudhakar1, T. Rama Krishnan2, J. Baghya Meena3, V. Shankar Ram4, H. Tamilzhelvan5, Kamal Kannadasan6, Parthiban7
1H.O.D and Professor, Thaimoogambigai Dental College and Hospital 2Professor, Meenakshiammal Dental College and Hospital 3Post Graduate, Thaimoogambigai Dental College and Hospital 4Reader, Thaimoogambigai dental College and Hospital 5Professor, Ramachandra Dental College and Hospital 6Professor, Thaimoogambigai Dental College and Hospital 7Senior lecturer, Thaimoogambigai Dental College and Hospital
ABSTRACT: The aim of the study was to compare the clinical efficiency of a diode laser as an adjunct to scaling and root planning in the treatment of chronic periodontitis patients A total of thirty patients with chronic periodontitis were selected for the study. Randomly 15 patients were taken in each group as control group and test group. The test group received conventional SRP and diode laser. The plasma, GCF & Saliva levels of reactive oxygen metabolites were estimated at baseline, day 30 & day 60 for test group When the groups were compared statistically significant reduction was seen in reactive oxygen metabolite from baseline to day 30 and to day 60(P<0.001) In this study it can be concluded that the use of diode laser as an adjunct to SRP provided significant result compared from baseline, day30 & day 60
KEYWORDS: Surgical Periodontal; Root planning; GCF; Salive; Periodontitis
Download this article as:| Copy the following to cite this article: Sudhakar U, Krishnan T. R, Meena J. B, Ram V. S, Tamilzhelvan H, Kannadasan K, Parthiban. Short Term Effects of Non Surgical Periodontal Treatment with the Use of Diode Laser(810nm) on Plasma, Gcf and Saliva Level of Reactive Oxygen Metabolite in Pateients with Chronic Periodontitis. Biosci Biotech Res Asia 2015;12(2) |
Introduction
Periodontitis is a inflammatory lesion, mediated by complex host parasite interactions, that leads to the loss of connective tissue attachment to root surface cementum and adjacent alveolar bone. The inflammatory and immune responses to the bacteria that colonize the periodontal and associated tissues involve the sytemic circulation and ultimately the peripheral system of the body
The neutrophils play a pivotal role in the host defense and are the first line of defense against these infectious periodontal diseases2. The difference of microbial products ( especially those produced by gram negative bacilli) triggers local inflammation and causes large members of Polymorpholeucocytes(PMNs) to migrate to the area where the bacteria interact with the surface tissue3. Reactive oxygen species (ROS) released from the PMNs and other immune cells lead to oxidative damage in biomolecules, which trigger morphofunctional changes in the endothelium and therefore in the periodontal tissues that nourish them. In normal physiology, there is a dynamic equilibrium between ROS activity and antioxidant defense capacity and when the equilibrium shift in level of ROS, either by reduction in level of antioxidant defenses or an increase in ROS production or activity, oxidative stress results. This imbalance between the ROS-AO has been implicated as one of the progressive or pathogenic factors for the periodontal diseases4. These oxygen derived free radicals are highly reactive molecular species that can disrupt cellular proteins, nucleic acids and membrane lipids as well as cause depolymerisation of matrix components such as collagen, hyaluronan and proteoglycans5.
Recent clinical studies suggest a positive association between periodontitis and oxidative damage6. A significantly higher level of serum proteins carboxyl( a marker of protein oxidation) was observed in patients with periodontitis than in periodontically healthy subjects.
A number of surgical and non surgical modalities are available for the treatment of inflammatory periodontal diseases. Subgingival scaling and root planning is one of the most important procedure and this efficancy has been demonstrated in numerous clinical studies7. Laser applications in periodontology have been of enormous interest in the recent years. The earliest studies mentioning the application of laser in the non surgical pocket therapy began in 1990s8.
Lasers may contribute to bacterial reduction in periodontal pocket as well as contribute to the removal of calculus and granulation tissue can also be used for debridement of soft tissue wall9. The high powered Carbondioxide(co2) and Neodymium Doped Yttrium Aluminium Garnet(Nd:YAG) lasers are capable of excellent soft tissue ablations with a good haemostatic effect and have been generally proposed for periodontal and oral surgery10. However, these lasers are not suitable for root surface treatment , because of the carbonization of these tissues and major thermal effects on adjacent tissues. Diode and NdYAG are currently used by the clinicians because of its delivery system which is convenient to use. A variety of diode lasers have been used in the debridement of periodontal pocket and a number in vitro and in vivo studies have be carried out. Studies have been done to evaluate the possible advantage of the use of diode laser as an adjuvant to conventional SRP.
The purpose of the present study was to determine the effects of the use of diode laser in pocket debridement and the changes in the ROM levels in plasma, saliva and GCF.
Materials and Methods
The study population consisted of 30 subjects ( Each group has 15 subjects) belonging to both sexes and all subjects were randomly selected from the outpatient clinic of the Department of Periodontics,Thaimoogambigai Dental college, Chennai. The patients were clinically and radiographically evaluated for CP. The patients had atleast 4 teeth with one (or) more sites exhibiting probing depth ≥ 4 mm. Clinical attachment level of ≥ 4 mm with radiographic evidence of bone loss. The control group consisted of individuals with probing depth ≤ 3 mm and clinical attachment level of ≤ 1 mm, with no clinical signs of gingival inflammation and who also maintained a good oral hygiene.
Among the criteria for being included in the study were patients having no systemic disease, having received no periodontal treatment, antibiotics, antinflammatory agents (or) other drugs in the last 6 months. The participants were informed about the study and their consent was obtained. The study protocol was approved by the ethical committee of Dr. M.G.R University Chennai, India, in accordance with the Declaration of 1975, as revised in 2000.
Group I-Healthy Periodontium
Group II- Chronic Periodontitis
Evaluation of Periodontal status
All data was recorded in a standard proforma. Oral examination was carried out with proper illuminations using mouth mirror and graduated Williams’s periodontal probe. The following parameters were evaluated for the subjects:
Plaque index by Loe and Silness (1963)
Probing Pocket Depth (PPD)and Clinical Attachment Level(CAL)
Collection of GCF
Detailed case history, clinical examination and supragingival scaling were done one day before the collection of GCF. On the subsequent day after drying the area with a blast of air, supragingival plaque was removed without touching the marginal gingiva and the GCF was collected. A standardized volume of 1µl was collected from each site with an extracrevicular approach , using volumetric capillary pipettes that were collected from 1-5µl. The collected GCF was transferred immediately to ependeroff tubes and stored at -70·c until the time of assay.
Collection of plasma
Blood (3ml) was drawn from the antecubital vein, under aseptic precautions and collected in a colour coded herparinised test tube ( green colour) and centrifuged immediately at 3000 xg for 5 minutes. If not immediately assayed plasma aliquots were stored at -80 c until analysis.
Collection of saliva
Draining/ Spitting method: The subject is asked to accumulate saliva in the floor of mouth and then spit in to a pre- weighed or graduated test tube.
Laboratory method for detection of ROM
The d- ROM test, developed by world- renowned Italian biochemist Mauro Carratelli, is a photometric test for measurement of the concentration of hydroperoxides (ROOH) in biological samples. The presence of ROOH in cells indicates oxidative attack of ROS on various substrates such as carbohydrates, lipids, amino acids, proteins, or nucleotides.
Test Principle
The d- ROM test uses the principle of Fenton’s reaction: by mixing a biological sample with an acidic buffer (Reagent R1), the newly- created transition metal ion (ion or copper) catalyzes the breakdown of hydroperoxide, generating new radical species such as hydroxyperoxyl (ROO+) and aikoxyl (RO+). By adding a chromogen (N,N- diethyl- paraphenylendiamine, Reagent R2) having the ability to donate the electron and change color when oxidized by free radicals, and using photometric reading available with the FRAS 4 dedicated analytical equipment, it becomes possible to quantify the level of hydroperoxides available in the sample.
Periodontal Treatment
At baseline, the clinical parameters(Plaque index, Gingival index, Probing Pocket depth ,clinical attachment level ) were recorded for all subjects and full mouth supragingival scaling was done. GCF and plasma samples were collected for assessment of ROM levels. No treatment was done after sample collection in Group I patients. In Group II patients, Non-surgical periodontal treatment (Scaling and Root planing )was performed .On completion of scaling and root planing low level laser therapy was performed using diode laser.The treatment consisted of an average of four 45 minute sessions over 3 weeks. Clinical data ,GCF, saliva and plasma samples were reevaluated at 1st & 2 months after therapy in group II patients to assess the changes.
Laser parameters
On completion of scaling and root planning, full mouth low level laser therapy was done using a diode laser. Picaso laser is a class IV diode laser, with an active semiconductor medium (Gallium,Aluminium, Arsenide) with a wavelength of 810nm provided with a optical fiber thickness of 300µm. The laser was fired at the orifice of gingival margin at a distance of 1cm in a non contact mode using a setting of 1.5 W as a continuous wave. Each tooth received 5-10s of exposure. Final low level laser therapy was done on 2nd day. Low level laser therapy was performed two times at baseline and 2nd day . Protective shields were worn by the operator, assistant and patient during the entire procedure.
Statistical analysis
Statistical analyses were performed using a soft were program ( SPSSV 16, IL ).Intependent t test was used for comparision of ROM levels in GCF, saliva & plasma among healthy & chronic periodontitis. Comparision of ROM levels between baseline and after treatment was done using repeated measure ANOVA
Results
All 30 Patients attended baseline and follow up appointments. At the baseline, there was significant difference in ROM levels between healthy and chronic periodontitis patients ( p value < 0.001) in GCF, Saliva & Plasma. This is depicted in table 1.
Table 1: Comparison of the ROM levels in GCF, PLASMA AND SALIVA among healthy and chronic periodontitis patients
| Group | N | Mean | Std. Deviation | Std. Error Mean | P value | |
| GCFROM | Healthy | 15 | 267.68 | 78.975 | 20.39 | <0.001 |
| CP | 15 | 484.33 | 136.59 | 35.26 | ||
| PLASMAROM | Healthy | 15 | 300.37 | 37.77 | 9.75 | <0.001 |
| CP | 15 | 596.00 | 46.77 | 12.07 | ||
| SALIVAROM | Healthy | 15 | 321.64 | 49.96 | 12.90 | <0.001 |
| CP | 15 | 596.00 | 46.77 | 12.07 |
Comparision of ROM levels GCF in the test group at different time point (30 days+ 60 days ) was depicted in table 2. The mean changes in GCF levels of ROM were statistically significant between baseline and day 60 and between day 30 and 60 in the test group ( p< 0.001)
Table 2: Comparison of ROM in GCF at baseline and after laser treatment at 1 and 3 month interval
| ROM-GCF | Mean | Std. Devi | 95% Confidence Interval | P value | |
| Lower Bound | Upper Bound | ||||
| Baseline | 484.333 | 136.59840 | 408.688 | 559.979 | <0.001 |
| 1 month | 387.733 | 137.33089 | 311.682 | 463.785 | |
| 3 months | 341.467 | 138.18146 | 264.944 | 417.989 | |
Comparision of ROM levels in plasma after laser treatment at different time points ( 30 & 60 days) was detected in table 3. There was significant difference in ROM levels in plasma between baseline and day 60 and between day 30 and day 60 ( p < 0.001)
Table 3: Comparison of ROM in Plasma at baseline and after laser treatment at 1 and 3 month interval
| ROM_PLASMA | Mean | Std. Dev | 95% Confidence Interval | P value | |
| Lower Bound | Upper Bound | ||||
| Baseline | 596.001 | 46.77981 | 570.095 | 621.906 | <0.001 |
| 1 month | 499.867 | 46.62705 | 474.046 | 525.689 | |
| 3 months | 455.667 | 46.19850 | 430.083 | 481.251 | |
Comparision of ROM levels in saliva at baseline and after treatment at different timepoints ( day 30 + day 60) were depicted in table 4. There was significant difference in ROM levels in saliva between baseline and 60th day and between day 30 and day 60 ( p value < 0.001)
Table 4: Comparison of ROM in Saliva at baseline and after laser treatment at 1 and 3 month interval
| ROM_SALIVA | Mean | Std. Dev | 95% Confidence Interval | P vlaue | |
| Lower Bound | Upper Bound | ||||
| Baseline | 596.001 | 46.77981 | 570.095 | 621.906 | <0.001 |
| 1 month | 503.667 | 47.38979 | 477.424 | 529.911 | |
| 3 months | 456.734 | 46.97311 | 430.721 | 482.747 | |
None of the patients reported any side effects releated to laser radiation
Discussion
To the best of our knowledge, this is the first study performed to estimate the levels of ROM in saliva, GCF & plasma in patients with chronic periodontitis undergoing SRP with Low level laser therapy (LLLT)
Recently, the use of laser in the dental field is more pronounced. Today low level laser are used to improve wound healing 11. Low level laser therapy are thought to act by the intraction of light with the cell & tissue. The dose applied during laser applications is one of the important treatment parameter to low level laser therapy. However, a precisely determined dose has not been proved for each indication. Biostimulation has been reported in the literature with doses between 0.001 and 10 J/cm3 as a theraputive window12. Mester et al 13 suggested in 1971 that doses of ≈1 K 2J/cm3 are necessary to see an effect on wound healing. In this study we used diode laser of 810 nm out put power of 1 w.
The results of our study show that there is statistically significant improvement in ROM levels and clinical parameters post treatment. The beneficial effects of scaling and root planing combined with personal plaque control in the treatment of periodontitis have been well documented14.. Low level laser therapy(LLLT) after non surgical periodontal treatment resulted in more reduction in clinical parameters. The improvement could be a result of an increase of anti- inflammatory cytokine levels & an increase of microcirculation by LLLT.
The levels of ROM reduced from 460 (GCF), 562.5(plasma), 562.5(Saliva) caaru to 360, 467.5, 467.5 in day 30 and they reduced to, 315, 417.5, 422.5 to day 60 in test group respectively. The reduction in levels of ROM from baseline to day 30 and from baseline to day 60 was found to be statistically significant (p < 0.001). This was in accordance with previous studies that demonstrated statistically significant decrease in levels of ROM in patients with chronic periodontitis as compared to control before & after treatment 16,17. The reduction of ROM after laser bedridement was initially reported in an in vitro study in which attenuation of ROM production in neutrophils was demonstrated by low level laser therapy18,19. However, certain previous reports have demonstrated that periodontal treatment had minimal influence on the plasma levels of total antioxidants20. The present study provide some support that periodontitis is the common cause of low grade inflammation that can lead to a systemic oxidative stress state. The excessive circulating ROS thus produced can oxidize DNA, lipids and proteins thereby contributing to tissue damage. So, it can be said that periodontitis induced high oxidative stress can lead to similar tissue damage. It could be said that, the reduction of circulating ROS by periodontal treatment offers clinical benefit.
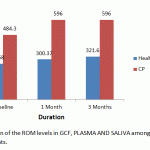 |
Figure 1: Comparison of the ROM levels in GCF, PLASMA AND SALIVA among healthy and chronic periodontitis patients |
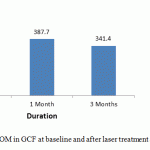 |
Figure 2: Comparison of ROM in GCF at baseline and after laser treatment at 1 and 3 month interval |
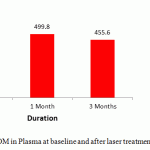 |
Figure 3: Comparison of ROM in Plasma at baseline and after laser treatment at 1 and 3 month interval |
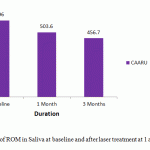 |
Table 4: Comparison of ROM in Saliva at baseline and after laser treatment at 1 and 3 month interval |
Conclusion
The result of this study, show that non surgical therapy of periodontal patient using diode laser as an adjunct had beneficial results. There was a significant reduction at various time points. Limitations of the present study, include small sample size and results are not compared with other treatment modalities. Further long term studies are required with large sample size to evaluate the effect of LLLT as an adjuvant to SRP in the treatment of periodontal patients and their effects on Oxidative stress.
Bibilography
- Consensus report periodontal diseases, pathogenesis and microbial Ann. Periodontal 1996, 926-932.
- Dennison D.K & T.E. Vandyke, The acute inflammatory response & the role of phagocyte cells in periodontal disease and health. Perio 2000, 1997; 14; 54-78.
- Batting M, Bullon P, Wilson M, Neumen H. Oxidative injury & inflammation in periodontal diseases, the challenge of antioxidants to free radicals and reactive oxygen species. Crit Rew oral Biol med; 10; 458-475.
- Waddington R, R.Moseley and Embery. Periodontal diseases mechanism: Reactive oxygen species a potential role in the pathogenesis of periodontal diseases, Oral diseases. 2000;6(3);138-151.
- Akalin FA, Baltaiciogli F, aloer A: Lipid periodoxidation levels and total oxidant status in serum, saliva and GCF in patients with chronic periodontitis. J clinical periodontology 2007; 34; 558-565.
- Battacioghi f, Akalin FA, Alver A, Deger O, Karabulour F. Protein carboxyl levels in serum and GCF in patients with chronic periodontitis. Arch oral boil 2008; 53; 716-722.
- Badersten A, Nilenus R, Eglberg J. Effect of non surgical therapy in moderately advanced periodontitis. J clin periodontology 1981; 8; 57-72.
- Trylovich PJ, Cobb CM, Pippin P, Spencer P, Kiley W. The effects of NdYAG laser in vitro fibroblast attachment in endotoxin treated root surface. J Periodontal 1992; 63; 626-632.
- Kesler M, Haqha: Clinical efficacy of semiconductor laser application as an adjunct to conventional SRP. Laser Surg med; 2005;37; 360-365.
- Aoki a, Sasaki K.M, Watenabe M, Khikawaj: Lasers in non surgical periodontal therapy. Perio 2000; 2004; 36; 59-97.
- Posten W, Wrone DA, Dover J.S, Amdt KA, Silapent .S, Alem M. Low level laser therapy for wound healing mechanism and efficacy. Dermatol surg 2005, 31 : 334- 340.
- Tuner J, Hude L. Some basic laser physics. The laser therapy handbook. Grangesberg. Sweden: prime books; 2007: 317- 338.
- Mester E, Spiry T, Szende B, Tote S.C. Effect of laser rays on wound healing. Am J Surg 1971; 122: 532-535.
- Hugher T.P, Caffusse RG. Gingival changes following SRP & oral hygiene. A biometric evaluation. J Periodontol 1978; 49 : 245- 252.
- Woodruff LD, Boukkeo JM, Bannon W.M et al . The efficacy of laser therapy in wound repair: A metaanalysis of literature. Photoned laser surg 2004; 22 : 241- 247.
- Tamaki N, Tomofuji T, Marnyama J et al. Short term effects of non surgical periodontal treatment on plasma levels of ROM in patients with chronic periodontitis. Journal of periodontal 2009; 80: 901- 906.
- Tamaki N, Tomofuji T, Montia Y. Periodontal treatment decrease plasma oxidised LDL level & oxidative stress. Clin oral investing 2011; 15, 953-958.
- Fujmaki Y, Shimoyema T, Liu Q, Umeda T, Nakaji S. Low level laser radiation attentuates production of ROS by human neutrophil. Laser surg med, 2003; 21; 165-170.
- Aarthi S. Bala subramaniam, Lobby J. Thomas, T.Ramakrishnan, N.Ambalavanan. Short term effects of non surgical periodontal treatment with & without use of diode laser on serum levels of ROM and clinical periodontal parameter in patients with chronic periodontitis. Quiescence international periodontology; 16-24.
- Chapple II, Brock G.R, Milward M.R, Ling N, Mathew.IB- compromised GCF total antioxidant capacity in periodontitis. Cause (to) effect? J.clinical periodontology 2007, 34; 103-110.

This work is licensed under a Creative Commons Attribution 4.0 International License.





