How to Cite | Publication History | PlumX Article Matrix
Elena Yu. Toropova1, Albina A. Kirichenko1, Grigory Ya. Stetsov2, Valery Y. Suhomlinov3
1Novosibirsk State Agrarian University (NSAU), 160, Dobrolubova Str., Novosibirsk, 630017, Russia 2Altai Research Institute of Agriculture, 35, Research town, Barnaul, 656910, Russia 3Novosibirsk State Agrarian University (NSAU), 160, Dobrolubova Str., Novosibirsk, 630017, Russia
ABSTRACT: The aim of the research was to study the effect of resource-saving technologies in the cultivation of spring wheat and barley in several regions of Western Siberia on the distribution and taxonomic composition of root rot pathogens, as well as the evaluation of protective measures efficiency. In the context of the resource-saving technologies of crop cultivation in Western Siberia, root rot became of particular relevance; the development of root rot exceeds the thresholds by up to 5-7 times, especially in the first half of the growing season. We identified substantial changes in the taxonomic composition of the pathogen complex of root rot towards expansion of the plant pathogens range by fungi of the Pythium genus and strengthening of the Fusarium genus fungi domination. There is a tendency of the ecological niches of plant pathogens spreading in organs of plants: B.sorokiniana better adapted to topsoil organs and the Fusarium genus fungi – to the underground ones. We identified environmental factors with the greatest impact on strengthening and spreading of the root rot pathogenesis: high number of intra-stem pests, which exceeds the threshold by 2-3 times, contamination of crops with cereal weeds – reserves of root rot pathogens, as well as low microbiological activity and soil suppressive qualities caused by a short growing season and lack of plant residues. We studied the efficiency of improvement methods for the phytosanitary condition of soil and underground plant organs of spring wheat and barley through the introduction of phytosanitary crop rotation and preceding crops, seed treatment and technologies to improve plant resistance in the initial phases of development.
KEYWORDS:
Resource saving; no-till; pathogenic micromycetes; root rot; technologies; previous crops; soil suppressive qualities; soil disinfectants
Download this article as:| Copy the following to cite this article: Toropova E. Y, Kirichenko A. A, Stetsov G. Y, Suhomlinov V. Y. Soil Infections of Grain Crops with the Use of the Resource-Saving Technologies in Western Siberia, Russia. Biosci Biotech Res Asia 2015;12(2) |
Introduction
West Siberia (Russia), along with other regions of the globe, refers to the zones of risk farming, where the cultivation of traditional crops, among which spring wheat and barley take a special place, is associated with the probability of yield losses due to abiotic (drought, frost, etc.) and biotic (pests) stressors. The current phase of agricultural development in arid zones is characterized by two features that are relevant to plant protection: farm specialization on cultivation of one culture (monoculture) without balanced crop rotations and tillage minimization (Wildermuth, & McNamara, 1984; Chulkina, 1985; Blackshaw,1991, 2005; Toropova, 2005; Schroeder, & Paulitz, 2006). Resource-saving technologies of crop cultivation are implemented on 30-50% of the area of Siberia, but direct seeding (No-till) occupies at the moment not more than 5% of the area. Gradually, the trend of resource saving expands, and according to the experts, by 2020 about 30% of arable land will be cultivated with the no-till technology.
In the current development phase of agricultural production in many regions of the world, including Siberia, one of the most common and harmful groups of crop diseases is root rot, which annually reduces yields by 25% or more (Wildermuth, 1986; Mergoum, 1993; Bailey, & Lazarovits, 2003; Razina, et al., 2008; Demina, & Kincharov, 2010; Toropova, et al., 2013; Hajihassani, et al., 2013). Under the influence of root rot, thinning, growth inhibition, the dynamics violation of plant organogenesis occur, the formation of backbone elements of the yield structure deteriorates, the quality of products greatly reduces, there is a possibility of its contamination with the toxins of plant pathogens (Verma, 1974; Chulkina, 1985; Labbe, 2002; Toropova, 2005; Bernhoft, et al., 2010).
The etiology of root rot in Siberia was determined in 70-80s of the 20th century and represents a complex of species, with the dominance of the most pathogenic fungi – Helminthosporium sativum (syn. Bipolaris sorokiniana) and species of the Fusarium genus (F.avenaceum, F.oxysporum, F.graminearum, F.sambucinum et al.), which have wide biocenotic communications and parasitize both on cultural and weedy cereal plants (Chulkina, 1985). In recent years, the number of economically important pathogenic micromycetes – causative agents of root rot in Siberia were classified as fungi of the Pythium and Rhizoctonia genera, which corresponds to international patterns (Paulitz, et al., 2002; Schroeder, et al., 2006; Morita, & Tojo, 2007).
Monitoring of spring wheat root infections in the last 10-15 years indicates gradual changes in the number of soil micromycetes colonisations, the nature and extent of ecological niches, change in the dominant species in mycocenosises, increase in virulence and aggressiveness of previously little pathogenic groups of organisms (Blackshaw, 2005; Feng, et al., 2003; Gardiner, et al., 2012; Bernhoft, et al., 2012). The reasons for colonization shifts are various and associated with the changes in the technologies of agricultural crop cultivation, breeding of new varieties, climatic variations (Schroeder, & Paulitz, 2006; Bernhoft, et al., 2012). In view of the above, it is especially important to monitor the taxonomic composition of root rot pathogens systematically in order to identify colonization shifts of dominant components of pathogenic mycocenosis, improve disease prognosis and optimize protective measures.
Methods for monitoring soil plant pathogens are constantly improving, including appearance of highly accurate immuno-genetic methods. This contributes to the development of more effective prognosis systems and protective measures, acceleration of the selection of resistant varieties (Leclerc-Potvin, 1999; Kumar, et al., 2002; Paulitz, & Schroeder, 2005; Schroeder, et al., 2006; Morita, & Tojo, 2007; Tyryshkin, 2010; Leng, et al., 2011). Since soil plant pathogens formed the K-strategy signs of life cycles in the evolution process, plant protection systems against them are aimed at reducing the initial colonisations of pathogens, factors of transmission in time (soil, seeds, infected plant residues) and at increasing the resistance of plants to root rot, especially in the initial critical periods of plant ontogenesis (Toropova, 2005).
The complex of protective measures against root rot universally includes phytosanitary crop rotation and previous crops, increase in soil suppressive qualities by introducing organic and mineral fertilizers, seed improvement techniques, especially treatment with chemical and biological fungicides, as well as measures to establish an effective seed bed for susceptible crops, including optimal seeding depth, pre- and post-sowing soil preparation, introduction of starting doses of nitrogen and phosphate fertilizers, and others (Chulkina, 1985; Wildermuth, & McNamara, 1991; Bailey, & Lazarovits, 2003; Toropova, 2005; Xiangsheng, et al., 2006; Fernandez, et al., 2007).
Agroecological features of the crop cultivation in the regions of various countries determined the taxonomic composition of plant pathogens, varietal characteristics of crops, cultivation techniques, composition, and the effectiveness of protective measures against root rot (Wildermuth, 1986; Van Leur, Bailey, 2000; Duveiller, & Altamirano, 2000; Stubbs, et al., 2004; Sieling, et al., 2005; Toropova, 2005; Fernandez, et al., 2008; Dixon, & Tilston, 2010; Karakulev, et al., 2011). Development of resource-saving technologies all over the world had a significant impact on the activity of a number of natural and anthropogenic factors that determine the epidemic process of root rot, which in turn required a detailed investigation (Grey, 1991; Bockus, & Shroyer, 1998; Bailey, & Lazarovits, 2003; Schroeder, & Paulitz, 2006; Paulitz, 2006; Dixon, & Tilston, 2010; Gardiner, et al., 2012).
The research aim was to study the effect of resource-saving technologies in cultivation of spring wheat and barley in several regions of Western Siberia on the distribution and taxonomic composition of root rot pathogens, as well as the evaluation of protective measures efficiency.
Methodology
The study had been conducted in the field conditions of forest-steppe zones of the Novosibirsk, Tomsk, Kemerovo, Omsk regions and the Altai Territory for 7 years (2007-2013). Annually we analysed 40-50 agrocenoses with the area of 100-350 hectares from 5-7 farms. Plant samples were collected at six points of the field in the phases of 2-3 leaves and full ripeness.
Phyto-examination of seeds was conducted by the following technique: seeds of spring wheat and barley were grown in moist rolls of filter paper in the dark. The total volume of the sample in the experiment – 100 seeds. Rolls were made from macroporous filter paper, pressed from both sides, 25cm wide and 50cm long, bend in half lengthwise. 50 seeds laid out in a roll (furrow on paper germ down), previously sterilized in a 0.5% solution of KMnO4 for 10-15 minutes and washed in running water until pink colour disappears, were covered with a strip of filter paper 2.5 -3.0 cm wide and 50 cm long. All paper was wetted with water until saturation. The rolls were incubated in an upright position in a sterile plastic bag for 7 days in the dark at 24-26˚C. Then the roll was unfolded, the top strip of filter paper was removed and the number of germinated seeds was counted. A seed was considered germinated, if its germ and roots were not less that 1cm. Then we counted a share of infected germs. A germ was considered infected, if it had brown spots or streaks on roots and shoot. After that, the germs were removed from the paper, and by the remaining spots on the paper we determined the proportion of germs infected with of the Fusarium genus fungi (pink colouration, characteristic sporulation) under a microscope. We looked through a bottom strip of the filter paper under a microscope and counted fungal colonies of B. sorokiniana and Alternaria spp. et al., microscoping under magnification of at least x100.
For mycological analysis of grain and ground organs of spring wheat and barley, we cut plant organs into equal 1 cm pieces on the artificial nutrient media, grain and plant pieces had been sterilized for 10 minutes in a 0.5% solution of KMnO4 and washed in running water until colouring disappeared then we spread 10 fragments (seeds) on agarized media on each petri dish. We accounted for micromycetes in 7 and 14 days, specifying the proportion of fragments (seeds), occupied by each taxon of fungi. We isolated micromycetes into a pure culture and identified species by classic determinants (Gerlach, & Nirenberg, 1982; Barnes, 1979; Simmons, 2007).
When accounting for root rot we differentiated by organs, using the following methodology: 100 plants dug out at 6 random points of the field. We carefully removed underground plant organs from the soil, thoroughly washed them in running water, and analysed primary roots, secondary roots, coleoptile, epicotyl, base of the plant by the following scale:
0 – an organ has a uniform light colour, healthy;
0.1 – there are small brown spots that take no more than 10% of the surface;
1 – darkened zone covers 25% of the surface;
2 – 50% of the organ surface is affected;
3 – 75% of the organ surface is affected;
4 – an organ is fully affected or dead.
After the analysis, we calculated the disease development, the prevalence of the disease in each organ and the average for a plant.
The analysis of the soil being colonised by B. sorokiniana conidia has been carried out for 22 years (1991-2013) by the flotation method (Chinn, & Ledingham, 1958). Soil samples were kilned to the air-dry state, then crushed and sifted through a soil sieve with openings of 1 mm. The sample weight of 10g was placed into a porcelain mortar and wetted with 1ml of tap water and thoroughly mixed with a spatula. We added 5 ml of vaseline oil and mixed the soil again, then transferred it into a stoppered cylinder with the capacity of 100 ml and added 49 ml of water. The resulting mixture had been shaken by vertical hand movements for 5 minutes, and then left to stand for 1.5-2 hours until a clear separation of liquids was seen. From the surface layer of the oil emulsion we pipetted 1 ml and transferred dropwise to a glass slide. Every drop had a volume of 0.025 ml. From each sample, we analysed at least 10 drops under a microscope at a magnification of 100 times and counted the number of pathogenic conidia. The resulting content of conidia was counted per 1g of air-dry soil.
To determine the pathogenicity and phytotoxicity of root rot pathogens, they were grown on the Capek liquid medium. After 10 days, prior to determining the toxicity, the culture liquid was separated from the mycelium by filtration. Sterilized seeds of barley had been soaked in culture liquid for 24 hours. Control seeds were soaked in water. After 24 hours of soaking, seeds were laid on filter paper, and rolls had been incubated for 7 days. The presence of phytotoxins in the culture liquid was determined by the growth of germinating capacity, length of roots and a seedling. Cultures were considered toxic if they caused not less than 30% growth inhibition in comparison with the control.
Statistical data processing by the dispersion and correlative analysis methods was carried out using STATISTICA and Microsoft Excel software packages. The reliability of differences was assessed by the Student’s test at the probability level of 95%.
The taxonomic composition of plant pathogens – root rot pathogens of cereal crops
The results of studies on spring wheat and barley root rots showed a significant development and prevalence of the disease both in the 2-3 leaf phase and by the end of the crops growing season (Table 1).
Table 1: Cereal crops affected by root rot in the forest steppe of Western Siberia (2007-2013), %
| Indicator (lim) | Summer wheat | Summer barley | ||
| 2-3 leaves | ripeness | 2-3 leaves | ripeness | |
| Disease development by organs on average | 4.1-20.6 | 12.8-54.9 | 5.6-35.5 | 18.9-70.2 |
| Prevalence of the disease | 18.6-56.4 | 52.7-100 | 18.4-59.9 | 87.6-100 |
On the majority (80%) of surveyed areas, the development and prevalence of root rot exceeded the damage thresholds in all years of research. In the seedling phase under favourable phytosanitary conditions (healthy or treated seeds, soil thinly colonised by phytopathogens) plants healthy on the outside were observed, but in the ripeness phase all underground organs had clear signs of significant affection by root rot. The excess of biological damage threshold (biological damage threshold (BDT) makes up 5% of disease development in the 2-3 leaf phase and 15% – in the phase of full ripeness), in the seedling phase it reached 5 times with wheat and 7 times with barley, which led to significant thinning of crops and inhibition of plants in the early phases of development. At the end of the growing season, BDT exceeded 3.6 and 4.7 times for wheat and barley, respectively. This had a negative impact not only on quantitative but also on qualitative indicators of seed and food grain.
The etiology of spring wheat root rot varies somewhat according to the phases of plant development (Figures 1, 2).
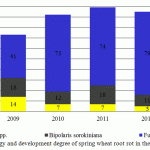 |
Figure 1: The etiology and development degree of spring wheat root rot in the tillering phase |
Thus, in the tillering phase of spring wheat plants, a pathogen complex was represented mainly by three groups of micromycetes: Bipolaris sorokiniana Sacc. Shoem., the fungi of the Fusarium genus and the fungi of the Pythium genus. However, the predominance of the Fusarium genus fungi needs to be noted. B. sorokiniana refers to traditional well-known and common pathogens, Fusarium and especially Pythium rots became widespread and economically important in the last decade. The major causative agent of Pythium wheat root rot in Western Siberia is the Pythium ultimum Trow. Pythium wheat root rot actively develops in cold and wet soil in the seedling phase.
At the end of the growing season, the prevalence of the root rot fungi of the Fusarium genus in the pathogenic complex becomes even more pronounced (Figure 2).
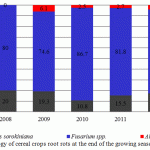 |
Figure 2: The etiology of cereal crops root rots at the end of the growing season |
The second most important fungi on the underground organs of spring wheat at the end of the growing season was Bipolaris sorokinianain, whose proportion in the pathogenic complex of root rot of wheat during the growing season could increase in wet years or decrease in more arid years. At the end of the growing season, on the underground organs we also found fungi of the Alternaria genus, the phytotoxicity study of which led us to the conclusion to include this taxon in the pathogenic complex of root rot causative agents. The study on more than 50 isolates of the Alternaria fungi showed that 45% of them exhibited strong phytotoxicity, and the dominant species both on cereal crops and on underground organs was Alternaria tenuissima, according PCR diagnostics (Nees et T. Nees: Fries) Wiltshire.
Fungi of the Fusarium genus, which dominated in the pathogenic complex of root rot, are dangerous because during transmission through seeds they can produce a series of mycotoxins dangerous for humans and animals, as a result cause the grain toxicosis (Bernhoft, et al., 2010, 2012; Kokkonen, 2012). When used for seed purposes, such grains may not come up or a plant, sprouted from an affected seed, will be weakened.
More than 10 species of the Fusarium genus annually parasitize on crops (Table 2).
Table 2: Incidence of Fusarium species on the organs of cereal crops in forest-steppes of Western Siberia (2007-2014)
| Type | Wheat | Barley | ||
| ear | underground organs | ear | underground organs | |
| F. sporotrichiella var. poae (Peck) Bilai (F. sporotrichioides Sherb.) | +++ | +++ | +++ | +++ |
| F. gibbosum Appel & Wollenw. (F. equiseti (Corda) Sacc.) | ++ | +++ | ++ | +++ |
| F. oxysporum Schltdl. | ++ | +++ | + | +++ |
| F. poae (Peck) Wollenw. | ++ | ++ | ++ | ++ |
| F. avenaceum. var. herbarum (Corda) Bilai (F. avenaceum (Fr.) Sacc.) | + | ++ | ++ | ++ |
| F. culmorum (W.G.Sm.) Sacc. | ++ | ++ | + | ++ |
| F. solani Koord. | + | +++ | + | ++ |
| F. moniliforme var. subglutinans Wollenw. & Reinking (F. subglutinans (Wollenw. & Reinking) P.E. Nelson, Toussoun & Marasas) | 0 | +++ | 0 | ++ |
| F. semitectum Berk. & Ravenel (F. incarnatum (Desm.) Sacc.) | + | + | + | + |
| F. heterosporum Nees. | + | ++ | + | ++ |
| F. lateritium Nees. | 0 | + | 0 | + |
| Fusarium spp. | + | +++ | + | ++ |
Note: “0” – not detected*, “+” – every 3-4 years, “++” – every 2 years, “+++” – every year.
The systematics is given by (Bilai, 1977), in brackets – by (www.wfcc.info/index.php/about/sites).
In all the regions, a significant proportion of the allocated fungi is accounted for highly aggressive species of F.oxysporum, F. sporotrichoides and F.moniliforme (F.subglutinans), F. gibbosum (F. equiseti), F. solani. It is important that in underground organs the diversity of fusaric fungi is always 2-3 times greater than on the ear that reflects their adaptation to the soil environment as the main ecological niche (Paulitz, et al., 2002; Dixon, & Tilston, 2010).
The development of root rot was determined primarily by the phytosanitary state of the soil (Table 3). The correlation coefficient between the number of plant pathogens propagules in the soil and the development of root rot was 0.955 ± 0.02, which means that it is the pest colonization of soil that determined the level of the disease by 91.2%, which fully reflects the evolutionary and ecological characteristics of soil plant pathogens, as K-strategists.
Table 3: The colonization with Bipolaris sorokiniana conidia of spring wheat soils in the farms of Siberia (1991-2013)
| Region
Territory |
Volume of analyses | Areas populated by phytopathogens, % | |||
| number of farms | thous. ha | lower that DT | higher that DT to the extent | ||
| moderate | high | ||||
| Omsk | 9 | 81.4 | 19.9 | 41.1 | 39.0 |
| Krasnoyarsk | 21 | 85.7 | 17.1 | 62.9 | 20.0 |
| Kemerovo | 6 | 26.2 | 10.6 | 71.4 | 18.0 |
| Novosibirsk | 21 | 258.2 | 6.8 | 66.1 | 27.1 |
| Tyumen | 3 | 4.6 | 9.1 | 51.5 | 39.4 |
| Altai | 26 | 242.1 | 3.6 | 49.2 | 47.2 |
| Sum, average | 86 | 698.2 | 11.2 | 57.0 | 31.8 |
According to the mass screening of arable soils in Siberia for Bipolaris sorokiniana conidia (about 700 thous. Ha were screened), 88.8% of the area under grain crops is colonized with pests above the damage threshold. It is one of the main prerequisites for epiphytoties of root rot in the farms of the Altai and Krasnoyarsk territories, Novosibirsk, Omsk, Kemerovo, Tyumen regions and leads to a drastic reduction in the yields of spring wheat and barley. Data shows that starting from the level of 200-300 B.sorokiniana conidias in 1g of air-dry soil, stabilization of the epiphytotic disease process occurs about at the same level, despite further growth of the phytopathogen colonization. At the same time, an important pattern is observed: a pathogen saturation level of ecological niches within the plant organs comes before the colonization stabilization in the soil. For spring wheat on a leached chernozem it is 200-300 and 500-600 conidia per gram of soil, respectively. The level at which the stabilization of the conidia colonization occurs, reflects the degree of natural suppressive qualities of soils of the region, and the stabilization level of the development index is also due to plants resistance to the disease, which depends on the genetic characteristic of varieties and a cultivation technology.
The role of environmental factors in the pathogenesis of root rot
Consideration of environmental factors that complicate the phytosanitary situation with soil pathogens suggests that low microbiological activity of zonal soils in Siberia plays an important role in these phenomena. This in turn is associated with a short and cold growing season (frost-free period is 90-130 days) and the lack of organic matter in soils. It was found that in the cold years the number of bacteria of all groups decreased by 2-4 times, the role of inadequate soil wetting during the growing season is even more significant.
A tillage system has a significant influence on microorganisms. The studies have shown that with tillage minimization, the number of microorganisms reduces by more than 10 times: with ploughing it amounts to hundreds of millions per gram, but with the minimum tillage and direct seeding, the number of bacteria decreases dramatically due to the fact that the soil becomes denser, cooler and micro-organisms do not always adapt to living in it.
The second essential environmental factor, which enhances the pathogenesis of root rot, is intra-stem pests: Oscinella frit L., O. pusilla Mg., Phorbia genitalis Schnb., Mayetiola destructor Say., the taxonomic composition of which is wide enough, which increases the overall harmfulness of the herbivores group, prolongs the period of them settling in the crops (Table 4).
Table 4: Colonization of cereal crops with intra-stem pests in the tillering phase in the Novosibirsk region, the research data on 12 agrocoenoses of four farms (2013-2014)
| Option | lim | Average | EDT |
| Wheat | 2.2-43.7 | 26.7 | 10% |
| Barley | 10.0-46.6 | 21.9 |
We determined a close correlation r = 0.98 ± 0.09 between damaged wheat stems with cereal flies and the development of root rot, especially on stem bases and coleoptiles of plants.
The third significant biotic factor, which influences the development of root rot in cereal crops, is grassy weeds. In recent years, certain facts were revealed, they suggest significant effect of crop infestation with cereal weeds on the development and harmfulness of root rots. Weeds are often the dominant component of phytocenoses, their number exceeds the density of wheat plants by up to 6.2 times. Competition with weeds reduced the resistance of plants, the development of root rot increased by 20% or more. Cereal weeds were also affected by root rots, contributing to the reproduction of plant pathogens. Seeds of cereal weed plants, which are the main source of cultivated soil infestation, served as a factor of root rot transfer, creating new and expanding already existing pockets of infection in the soil. The following pathogenic micromycetes were identified from the seeds of cereal weeds: Bipolaris sorokiniana (Sacc.) Shoemaker, representatives of the genera Alternaria Nees, Fusarium Link, Penicillium Link, Epicoccum Link, and others. The dominant taxon was the fungi of the Fusarium genus both on seeds and underground organs. We identified the following types of the Fusarium genus fungi from the roots of weed plants: F. sporotrichioides, F. avenaceum, F. equiseti, F. oxysporum, which are also widespread on underground organs of spring wheat and barley. The Jaccard coefficient of community of the species composition of the Fusarium genus fungi between underground organs of cereal weeds and spring wheat amounted to 0.55.
Among the natural abiotic factors that enhance the development and harmfulness of soil infections, dry growing conditions are of particular importance, and especially the June-July drought, which is typical for Western Siberia. On the contrary, the transfer of plant pathogens from seeds significantly intensifies in wet conditions of August, contributing to ear infection by airborne droplets.
Influence of the cultivation technology on root rot development
Technological methods had multidirectional influence on the development of root rot. Using the statistical analysis we determined a significant (62.1 … 89.2%) share of the influence of preceding crops on soil colonization with pathogens of root rot and disease progression, it significantly exceeded the impact of tillage, treatment and other anthropogenic factors (Table 5).
Table 5: The influence of preceding crops on soil colonization with Bipolaris sorokiniana conidia and root rot development in the forest-steppe of Western Siberia, 2007-2013
| Preceding crop | The density of conidia pcs/g of air dry soil | Share of degraded conidia, % | Root rot development on spring wheat, % | |
| Fluctuation limits | average | |||
| Summer wheat | 53-410 | 186 | 32 | 42.1 |
| Peas | 80-200 | 100 | 41 | 29.4 |
| Steam | 50-265 | 95 | 56 | 35.8 |
| Melilot | 40-130 | 68 | 62 | 24.7 |
| Vetch and oats | 25-65 | 45 | 67 | 17.5 |
| Barley | 110-526 | 215 | 23 | 54.1 |
| Corn | 80-125 | 100 | 47 | 37.2 |
| Perennial grasses | 20-40 | 30 | 74 | 11.3 |
| Total | 25-526 | |||
| *LSD05 | 27 | 2.1 | ||
*LSD05 – the least significant difference at the reliability level of 5% or at the probability level 95%
Introduction of phytosanitary crops, operating on the “germination-lysis” mechanism, into crop rotations led to the purification of soil from the propagules of plant pathogens. The best preceding crops are rape, clover, vetch-oat mixture, perennial cereal and leguminous grasses. They reduced the soil colonization with plant pathogens by 30% or more during 1 year, causing a massive degradation of resting structures of the phytopathogen.
Minimization of tillage had no effect on the colonization of plant pathogens, its role was limited to a concentration of plant pathogens in the upper layer and worsening of a phytosanitary situation during the germination phase and the seedling phase (Table 6).
Table 6: The development of root rot in spring wheat in the seedling phase with direct seeding in the stubble of a preceding crop in 2010-2013, %
| Organ | Development phase | НСР05 | |||
| Tillering | Ripeness | ||||
| absolute | DT number | absolute | DT number | ||
| Primary roots | 21.4 | 4.3 | 35.7 | 2.4 | |
| Colepoptile | 18.3 | 3.7 | 30.7 | 2.1 | |
| Axil of basal leaves | 15.3
|
3.1 | 30.1 | 2.0 | |
| Average per plant | 18.3 | 3.7 | 32.2 | 2.2 | 2.3 |
The data in Table 6 shows that in the early phases of ontogeny of spring wheat, which has been cultivated for more than 7 years with the No-till technology, the development of root rot exceeded DT by 2.9-4.4 times on average per plant only due to soil inoculum. The affection intensity of the underground organs of spring wheat varied. Primary roots were affected especially strong, it is associated with the concentration of conidia in the top layer of soil and unfavourable conditions during the seedling phase and the tillering phase. Colepoptile was affected strongly as well. In the phase of full ripeness, root rot development increased in all cases, however, the excess of DT was less significant compared to the seedling phase.
The stem base was affected particularly severely. This fact is connected with its damage during temperature and humidity changes in the compacted soil, as well as high concentration of pathogens in the topsoil. Thus, the transition to the direct seeding technology changes the seasonal EP dynamics of root rot so that the most urgent phytosanitary situation with No-till is formed in the seedling phase. This places special demands to quality and seed treatment, as well as to the creation of an effective bed for them. Localization of the main pathogens of root rot had peculiarities while minimizing tillage (Table 7).
Table 7: Localization of pathogenic micromycetes on underground organs of spring wheat with direct seeding (2010-2013 years)
|
The active ingredient, consumption rate, l/t of seeds |
Bipolaris sorokiniana | Fusarium spp. | ||
| laboratory on seeds | field, 3-4 leaves | laboratory on seeds | field, 3-4 leaves | |
| Difenoconazole + mefenoxam, 0.8 | 95 | 94 | 84 | 53 |
| Tebuconazole +
mefenoxam, 1.0 |
95 | 88 | 66 | 32 |
| Difenoconazole + cyproconazole, 1.0 | 90 | 82 | 81 | 36 |
| Tebuconazole, 0.25 | 80 | 72 | 74 | 38 |
| Thiabendazole + tebuconazole, 0.4 | 30 | 62 | 71 | 41 |
| Carboxin + thiram, 2.0 | 98 | 95 | 79 | 55 |
| Triticonazole, 0.2 | 85 | 82 | 72 | 30 |
| Triticonazole + prochloraz, 2.5 | 99 | 90 | 92 | 49 |
| Triticonazole + pyraclostrobin, 0.6 | 94 | 84 | 79 | 42 |
| Average | 93 | 82 | 72 | 33 |
*LSD05 – the least significant difference at the reliability level of 5% or at the probability level 95%Note the complete dominance of Fusarium genus fungi on primary roots and the epicotyl of spring wheat (77%); the secondary roots – 67%, on the stem base – 53%. Only on the stem base, Bipolaris sorokiniana amounted to a third of mycocenosis – 35.6 %. The Alternaria genus fungi were present in all organs of wheat in a small amount – 8.3-20.4 %. Most of them were on secondary roots and stem bases. Thus, with direct seeding by a stubble we identified a tendency to the divergence of ecological niches of the dark coloured B. sorokiniana fungus and the light coloured fungi of the Fusarium genus. B.sorokiniana is more often confined to topsoil organs and the Fusarium genus fungi – to the underground ones.Traditionally, according to the resource saving technology to disinfect seeds and protect seedlings from root rot pathogens, azole disinfectants are used, but the studies show that current drugs do not always cope with a wide taxonomic composition of pathogens (Table 8).
Table 8: The biological efficacy of seed treatments against the root rot pathogens on average in the regions of Western Siberia, 2008-2013, %
|
The active ingredient, consumption rate, l/t of seeds |
Bipolaris sorokiniana | Fusarium spp. | ||
| laboratory on seeds | field, 3-4 leaves | laboratory on seeds | field, 3-4 leaves | |
| Difenoconazole + mefenoxam, 0.8 | 95 | 94 | 84 | 53 |
| Tebuconazole +
mefenoxam, 1.0 |
95 | 88 | 66 | 32 |
| Difenoconazole + cyproconazole, 1.0 | 90 | 82 | 81 | 36 |
| Tebuconazole, 0.25 | 80 | 72 | 74 | 38 |
| Thiabendazole + tebuconazole, 0.4 | 30 | 62 | 71 | 41 |
| Carboxin + thiram, 2.0 | 98 | 95 | 79 | 55 |
| Triticonazole, 0.2 | 85 | 82 | 72 | 30 |
| Triticonazole + prochloraz, 2.5 | 99 | 90 | 92 | 49 |
| Triticonazole + pyraclostrobin, 0.6 | 94 | 84 | 79 | 42 |
| Average | 93 | 82 | 72 | 33 |
Analysis of the table shows that seed treatments, which are widely used in agricultural practice, showed high (80-95%) laboratory efficacy against B.sorokiniana. The exception was the thiabendazole + tebuconazole drug, the effectiveness of which under laboratory conditions was low and in some cases – negative. In an about half the experiments, thiabendazole + tebuconazole did not inhibit, but stimulated sporulation of dark-coloured fungi, and this phenomenon requires further study. The field efficacy of treatments against B.sorokiniana was slightly lower than the laboratory one, but high enough to deter the development of Helminthsporium root rot before tillering at a level close to the damage threshold.Seed treatments showed a relatively good (45-81%) efficacy in the laboratory against Fusarium root rot. In the phase of full shoots – the beginning of tillering, biological efficacy of the drugs was low (15-45%). As a result, in the field conditions the ecological niche of B.sorokiniana in the underground organs of crops seedlings was replaced by the fungi of the Fusarium genus, which exhibited resistance to treatments. According to the preliminary data, F. oxysporum and F. heterosporium showed resistance to tebuconazole, F. gibbosum (F. equiseti) and F. sporotrichoides – to triticonazole, but this information requires a detailed inspection. Thus, currently in order to protect seedlings of cereal crops from the Fusarium genus fungi, which are dominant in the pathogenic root rot complex, it is relevant to develop a more effective seed treatment.
Discussion
In the context of resource-saving technologies and crop cultivation in Western Siberia, the problem of root rot has become particularly relevant, as root rot causes significant loss of crops and the deterioration of product quality. In the taxonomic composition of the root rot pathogen complex, significant changes occurred towards expansion of the plant pathogens range by fungi of the Pythium genus and strengthening of the Fusarium genus fungi domination. There is a tendency of the ecological niches of plant pathogens spreading in organs of plants: B.sorokiniana better adapted to the near-Earth organs and fungi of the Fusarium genus – to the underground ones. We think that the reasons for these shifts are the changes in the soil conditions towards soil compaction, decrease in its oxygen content, limitation in microbiological activity, which create advantages for fungi of the Fusarium genus, as well as in broad application of seed treatments, the effectiveness of which is considerably higher against B.sorokiniana, resulting in the replacement of its environmental niche by Fusarium fungi in the early phases of the growing season (Korzhov, et al., 2009; Parikka, et al., 2012; Toropova, et al., 2013). Perhaps, the systematic use of pre-sprouting herbicides, increasing the susceptibility of plants to Fusarium, contributes to these processes (Fernandez, et al., 2009; Johal, & Huber, 2009). The development of root rot is more pronounced under the conditions of the resource-saving technologies in the early phases of plant development, because of the concentration of propagules of plant pathogens in the topsoil.To improve the situation, efforts should be aimed at improving the phytosanitary state of the soil through the implementation of the complex of agronomic (phytosanitary crop rotations, preceding crops, the system of organic and mineral nutrition) and operational (seed treatment) measures within the systems of integrated plant protection. Above measures will improve the phytosanitary situation both regarding intra-stem pests and cereal weeds, which enhance the pathogenesis of root rot, increase microbiological activity and suppressive qualities of soil (Dill-Macky, & Jones, 2000; Buyer, et al., 2002; Bailey, & Lazarovits, 2003; Kryukova, et al., 2011). Pesticides should also be applied against these groups of pests, especially during the transition period to the resource saving technologies.With the use of resource-saving technologies it is particularly important to increase the physiological resistance of plants to root rots in the seedling phase in order to neutralize the high concentration of propagules of plant pathogens in the topsoil and low microbiological activity of soil at low temperatures of air and soil in the planting period. To do this, it is necessary to create an effective seedbed based on their phyto-examination, with the results of which the requirements of plants are determined. Parameters of an effective seedbed: soil moisture is at least 60% of total moisture capacity, warming to + 4-5° C at a seeding depth, oxygen access, seed placement on a solid bed with unbroken capillaries, seed embedment into the soil not deeper than the average coleoptile length of a variety, which is determined for each batch of seeds, as this parameter is subject to changes from year to year, even of the same variety and, of course, it varies by varieties both of wheat and barley. Many modern disinfectants shorten a coleoptile length, depending on the active ingredient. It is possible to determine the effect of a particular disinfectant on seeds of a particular variety via test treatment during phyto-examination.To increase the resistance of plants to root rot, it is very important to control the content of mobile phosphorus in soils, especially in the initial phase of plant development (first 4-5 weeks). Despite the fact that in many soil types of Western Siberia this macro element is in excess, at low temperatures during seeding it is usually in the fixed form and plants do not receive it in sufficient quantities. With application of phosphate and potash fertilizers in rows at sowing, plants become more resistant to all soil pathogens (Chulkina, 1985; Ritchie, 1999; Xiangsheng, et al., 2006; Belyaeva, 2013).
Conclusion
The conducted research allowed revealing the main phytosanitary problems that agricultural producers in Siberia face during the transition to resource-saving technologies, and especially to direct stubbling-in of previous crops. These problems have significant similarities in different regions of the world, but certain problems have been identified that are specific only to Siberian regions, especially due to both environmental factors as well as economic and domestic economic circumstances. It is necessary to conduct detailed studies on biological and pathogenic features of the new dominants of pathogenic soil mycocenosis, to identify the most effective methods of monitoring, to assess the cumulative impact of resource-saving technologies on the state of soils, both by environmental and phytosanitary parameters. An important area for future research is to find out the exact causes of colonization shifts in the pedocoenosis of Western Siberian soils and the determination of the relative contribution of each of the identified natural and anthropogenic factors to the complexity of the soil infections problem. Ecologization of the cultivation technologies of agricultural crops with the activation of the environmental (natural) self-regulation mechanisms of the agrocoenosis phytosanitary state should be borne in mind as a prospect of future research in order to have cost-effective production of environmentally safe products competitive in the domestic and foreign markets.
References
- Chulkina, V., & Kondratiev R. (Ed.). (1985). Root rot of cereals in Siberia. Novosibirsk: Science, Siberian Branch.
- Toropova, E., & Chulkina V. (Ed.). (2005). Ecological bases of plant disease protection in Siberia. Novosibirsk.
- Toropova, E., Kazakova, O., Vorobeva, I., & Selyuk, M. (2013). Fusarium root rot of cereal crops in Western Siberia and the Transurals. Plant Protection and Quarantine, 9, 23-26.
- Verma, P. (1974). Studies of common root rot (Cochliobolus sativus) in manitou wheat: epidemiology, loss assessment, inoculum density, and effects of phosphate.
- Demina, E., & Kincharov, A. (2010). Pathogenicity and damage from root rot pathogens of wheat in the Samara region. Plant Protection and Quarantine, 11, 23-24.
- Hajihassani, A., Maafi, Z., & Hosseininejad, A. (2013). Interactions between Heterodera filipjevi and Fusarium culmorum, and between H. filipjevi and Bipolaris sorokiniana in winter wheat. Journal of Plant Diseases and Protection, 2(120), 77-84.
- Mergoum, M. (1993). Effects of infection by Fusarium acuminatum, Fusarium culmorum, or Cochliobolus sativus on wheat.
- Bailey, K., & Lazarovits, G. (2003). Suppressing soil-borne diseases with residue management and organic amendments. Soil & Tillage Research, 2(72), 169-180.
- Grey, W. (1991). Effects of environmental factors on infection of barley by parasitic or symbiotic soil-borne fungi.
- Karakulev, V., Glinushkin, A., & Solovykh, A. (2011). Phytosanitary features of spring wheat cultivation by terrain mesoforms on ordinary chernozems of the Orengburg region. Proceedings of the Orenburg State Agrarian University, 32-1(4), 66-68.
- Gardiner, D., Covarelli, L., Rusu, A., Kazan, K., Chakraborty, S., Manners, J., McDonald, M., McDonald, B., Solomon, P., & Marshall, M. (2012). Comparative pathogenomics reveals horizontally acquired novel virulence genes in fungi infecting cereal hosts. PLoS Pathogens, 9(8), e1002952.
- Tyryshkin, L. (2010). Possibility of using in vitro selection for obtaining wheat genotypes, resistant to bipolaris sorokiniana (sacc in sorok) shoem. Proceedings of the St. Petersburg State Agrarian University, 19, 39-43.
- Xiangsheng, L., Jiachen, W., Jun, Y., Yubin, F., Yanping, W., & He, Z. (2006). Application of rare earth phosphate fertilizer in western area of china. Journal of Rare Earths, 1(24), 423-426.
- Leng, Y., Liu, Z., Rasmussen, J., Zhong, S., Wu, C., & Friesen, T. (2011). RNA-mediated gene silencing in the cereal fungal pathogen Cochliobolus sativus. Molecular Plant Pathology, 3(12), 289-298.
- Leclerc-Potvin, C., Balmas, V., Charest, P., & Jabaji-Hare, S. (1999). Development of reliable molecular markers to detectnon-pathogenic binucleate rhizoctonia isolates (AG-G) using PCR. Mycological Research, 9(103), 1165-1172.
- Feng, Y., Motta, A., Reeves, D., Burmester, C., Van Santen, E., & Osborne, J. (2003). Soil microbial communities under conventional-till and no-till continuous cotton systems. Soil Biology and Biochemistry, 12(35), 1693-1703.
- Ritchie, K. (1999). Starter fertilizers for no-till and minimum-till corn.
- Belyaeva, O. (2013). No-till system and its impact on accessibility of soil nitrogen and fertilizers summary of the experiment. Agriculture, 7, 16-18.
- Stubbs, T., Kennedy, A., & Schillinger, W. (2004). Soil ecosystem changes during the transition to no-till cropping. Journal of Crop Improvement, 1-2(11), 105-135.
- Korzhov, S., Maslov, V., & Orehova, E. (2009). Changes in soil microbial activity with different ways of its treatment. Agro XXI, 1-3.
- Kryukova, E., Malanina, Z., & Kolmukidi, S. (2011). The role of grassy weeds, trees and shrubs in the infectious processes of agrarian and agro-forestry landscapes. Plant Protection and Quarantine, 4, 20 -23.
- Buyer, J., Roberts, D., & Russek-Cohen, E. (2002). Soil and plant effects on microbial community structure. J. Microbiol., 48, 955-964.
- Blackshaw, R. (2005). Tillage intensity affects weed communities in agroecosystems. Invasive Plants: Ecological and Agricultural Aspects (pp. 209-221). Switzerland: Burkhauser Verlag.
- Schroeder, K., & Paulitz, T. (2006, September). Root Diseases of Wheat and Barley During the Transition from Conventional Tillage to Direct Seeding. Plant Disease, 1247-
- Wildermuth, , Thomas, G., Radford, B., McNamara, R., & Kelly, A. (1997). Crown rot and common root rot in wheat grown under different tillage and stubble treatments in southern Queensland, Australia. Journal: Soil & Tillage Research – SOIL TILL RES, 3(44), 211-224.
- Wildermuth, G. (1986). Geographic distribution of common root rot and Bipolaris sorokiniana in Queensland wheat soils. Australian Journal of Experimental Agriculture – AUST J EXP AGR , 5(26), 601- 606.
- Wildermuth, G., & McNamara, R. (1991). Effect of cropping history on soil colonizations of Bipolaris sorokiniana and common root rot of wheat. Australian Journal of Agricultural Research – AUST J AGR RES, 5(42), 779-790.
- Fernandez, M., Zentner, , DePauw, R., Gehl, D., & Stevenson, F. (2007). Impacts of Crop Production Factors on Common Root Rot of Barley in Eastern Saskatchewan. Crop Science – CROP SCI, 4(47).
- Van Leur, J., Alamdar, M., & Khawatmi, S. (1997). Effect of common root rot (Cochliobolus sativus) on yields of barley under experimental conditions in northern Syria. Australian Journal of Agricultural Research, 48(3), 351-358.
- Bailey, K., & Lazarovits, G. (2003). Suppressing soil-borne diseases with residue management and organic amendments. Soil & Tillage Research – SOIL TILL RES, 2(72), 169-180.
- Sieling, K., Stahl, , Winkelmann, C., Christen, O. (2005). Growth and yield of winter wheat in the first 3 years of a monoculture under varying N fertilization in NW Germany. European Journal of Agronomy – EUR J AGRON, 1(22), 71-84.
- Fernandez, M., Huber, D., Basnyat, , & Zentner, R. (2008). Impact of agronomic practices on colonizations of Fusarium and other fungi in cereal and noncereal crop residues on the Canadian Prairies. Soil & Tillage Research – SOIL TILL RES, 1(100), 60-71.
- Johal, G., & Huber, D. (2009). Glyphosate effects on diseases of plants. European Journal of Agronomy – EUR J AGRON, 3(31), 144-152.
- Fernandez, M., Zentner, R., Basnyat, P., Gehl, , Selles, F., Huber, D. (2009). Glyphosate associations with cereal diseases caused by Fusarium spp. in the Canadian Prairies. European Journal of Agronomy – EUR J AGRON, 3(31), 133-143.
- Dill-Macky, R., & Jones, R. (2000). The Effect of Previous Crop Residues and Tillage on Fusarium Head Blight of Wheat. Plant Disease – PLANT DIS, 1(84), 71-76.
- Bernhoft, A., Torp, M., Clasen, P., Løes, , & Kristoffersen, A. (2012). Influence of agronomic and climatic factors on Fusarium infestation and mycotoxin contamination of cereals in Norway. Food Additives & Contaminants: Part A, 1-12.
- Bernhoft, , Clasen, A., & Kristoffersen, M. (2010). Torp Less Fusarium infestation and mycotoxin contamination in organic than in conventional cereals. Food Additives & Contaminants: Part A, 6(27), 842-852.
- Fernandez, M., Zentner, , DePauw, R., Gehl, D., Stevenson, F. (2007). Impacts of Crop Production Factors on Fusarium Head Blight in Barley in Eastern Saskatchewan. Crop Science – CROP SCI, 4(47).
- Parikka, P, Hakala, , & Tiilikkala, K. (2012). Expected shifts in Fusarium species’ composition on cereal grain in Northern Europe due to climatic change. Food Additives & Contaminants: Part A, 1-13.
- Bockus, , & Shroyer, J. (1998). The impact of reduced tillage on soilborne plant pathogens. Annual Review of Phytopathology – ANNU REV PHYTOPATHOL, 1(36), 485-500.
- Van Leur, J., & Bailey, K. (2000). The occurrence of barley root diseases in different agri-ecological zones of Syria. Canadian Journal of Plant Pathology-revue Canadienne De Phytopathologie – CAN J PLANT PATHOL, 1(22), 61-69.
- Duveiller, E., & Garcia, I. (2000). Pathogenicity of Bipolaris sorokiniana isolates from wheat roots, leaves and grains in Mexico. Plant Pathology – PLANT PATHOL, 2(49), 235-242.
- Kumar, J., Schafer, P., Huckelhoven, R., Langen, G., Baltruschat, H., Stein, E., Nagarajan, S., & Kogel, K. (2002). Bipolaris sorokiniana, a cereal pathogen of global concern: cytological and molecular approaches towards better control. Molecular Plant Pathology – MOL PLANT PATHOL, 4(3), 185-195.
- Schroeder, K., Okubara, P., Tambong, J., Lévesque, C., & Paulitz, T. (2006). Identification and quantification of pathogenic Pythium spp. from soils in eastern Washington using real-time polymerase chain reaction. Phytopathology, 96, 637-647.
- Paulitz, T, & Schroeder, K. (2005). A new method for the quantification of Rhizoctonia solani and R. oryzae from soil. Plant Dis., 89, 767-772.
- Schroeder, K., & Paulitz, T. (2006). Root diseases of wheat and barley during the transition from conventional tillage to direct seeding. Plant Dis., 90, 1247-1253.
- Paulitz, T. (2006). Low input no-till cereal production in the Pacific Northwest of the U.S.: the challenges of root diseases European Journal of Plant Pathology.
- Morita, Y., & Tojo, M. (2007). Modifications of PARP medium using fluazinam, miconazole, and nystatin for detection of Pythium spp. in soil. Plant Dis., 91, 1591-1599.
- Paulitz, T., Smiley, R., & Cook, R. (2002). Insights into the prevalence and management of soilborne cereal pathogens under direct seeding in the Pacific Northwest, U.S.A. J. Plant Pathol., 24, 416-428.
- Barnes, E. (1979). The Deuteromycetes: (The Fungi Imperfecti). Atlas and Manual of Plant Pathology (pp. 222-224).
- Chinn, S., & Ledingham, R. (1958). Application of new laboratory method for the determination of the survival of Helminthosporium sativum spores in soil. J. Bot., 3(36), 289-295.
- Dixon, G., & Tilston, E. (2010). Soil-Borne Pathogens and Their Interactions with the Soil Environment. Soil Microbiology and Sustainable Crop Production, 197-271.
- Gerlach, W., & Nirenberg, H. (1982). The genus Fusarium – a Pictorial Atlas. Biol. Bundesanst. Land-Forstw., Berlin-Dahlem, (209), 406.
- Kokkonen, M., Jestoi, M., & Laitila, A. (2012). Mycotoxin production of Fusarium langsethiae and Fusarium sporotrichioides on cereal-based substrates. Mycotoxin Research, 1(28), 25-35.
- Simmons, E. (2007). Alternaria. An Identification Manual. Utrecht: CBS.

This work is licensed under a Creative Commons Attribution 4.0 International License.





