How to Cite | Publication History | PlumX Article Matrix
Almaz Bahitovich Kaldybayev1, Birzhan Sardarovich Shakirov1, Raziya Adilbekovna Isayeva1, Kurmanbek Tazhmahanbetovich Zhantasov1, Mikhail Rodionovich Baklanov2
1Auezov South Kazakhstan State University, 160012 Republic of Kazakhstan, Shymkent city, Tauke khan, 5 2Interuniversity Micro-Electronics Center at Leuven, Kapeldreef 75, B-3001, Leuven, Belgium
DOI : http://dx.doi.org/10.13005/bbra/1931
ABSTRACT: This work is aimed at solving the problem on sorption purification of waste gases from sulfur dioxide on the wastes of phosphoric industry, i.e. thermophosphorus slags (TPhS). The solution of this problem is implemented by the new technical result, which is caused in increasing the depth of treatment by sulfur dioxide. Essential features of the claimed technical solution are the sorption purification of sulfur dioxide from waste gases on sorbent, containing layer of the granular TPhS. The experimental results showed that the highest activity of the sorbent, calcined at 500°C, is manifested at a temperature of sorption of 200 °C. With further increase of the process temperature, the degree of sorption does not change and duration of work of the sorbent is reduced.
KEYWORDS: Thermophosphorus slag; adsorption of SO2; catalysis; calcination; chemisorptions; activation of metal oxides
Download this article as:| Copy the following to cite this article: Kaldybayev A. B, Shakirov B. S, Isayeva R. A, Zhantasov K. T, Baklanov M. R. Adsorption-Catalytic Purification of Gas Mixtures From Sulphur Dioxide By Sorbent of Phosphoric Production Wastes. Biosci Biotechnol Res Asia 2015;12(3) |
| Copy the following to cite this URL: Kaldybayev A. B, Shakirov B. S, Isayeva R. A, Zhantasov K. T, Baklanov M. R. Adsorption-Catalytic Purification of Gas Mixtures From Sulphur Dioxide By Sorbent of Phosphoric Production Wastes. Biosci Biotechnol Res Asia 2015;12(3). Available from: https://www.biotech-asia.org/?p=3584> |
Introduction
In the modern period a treatment of emissions from harmful substances for the majority of industrial enterprises is one of the basic measures on protection of air basin. Neutralization of wastes supposes either removal of harmful impurities from inert gas-carrier or turning them into harmless substances. Both principles can be implemented through a variety of physical and chemical processes, implementations of which require certain conditions for the gas cleaning technology.
Due to the formation of large amounts as wastes, sulfur dioxide becomes one of the main gases that pollute the atmosphere. The greatest danger is the pollution by sulfur compounds which are released into the atmosphere at combustion of coal, oil and natural gas, as well as metal smelting and sulfuric acid production (Ling et al. 2010), which falls back to the earth in the form of acid rain. The acid rains have destructive effects on the biosphere (Jin-an et al. 1999).
The most promising, for the purification of waste gases from sulfur dioxide is the use of metals, especially alkaline earth or amphoteric metals oxides or carbonates as an adsorbent (Bennici et al., 2003; Smirnov et al., 2005; Bretschneider and Kurfurst, 1989; Li et al., 2004; Chmielarz et al., 2006). However, a special preparation of individual metal oxides significantly complicates and makes the process more expensive. That’s way, the use of more available and cheap natural or recycled materials for these purposes should be considered as beneficial and appropriate.
Currently, one of the acceptable methods of gas emissions purification from harmful impurities, in particular sulfur dioxide is the adsorption method with the use of phosphorus waste production, developed by the authors of this research.
Materials and methods
The objects and methods have been selected, the issues of purification of the flue gas emissions with the use termophosphorus slag have been considered to perform the research work.
The installation for carrying out of the research on purification of gas mixtures from sulfur dioxide on the basis of the laboratory has been designed and created.
The installation is intended for carrying out experiments on the purification of model gas mixture from sulfur dioxide (Figure 1). It consists of the following parts:
- a part for preparation of the model gas mixture;
- a part for carrying out the experiments on the purification of the gas mixture by solid sorbent.
The model gas mixture is prepared by mixing of gaseous sulfur dioxide, supplied from balloon (1) through a gasholder with layer of oil, by preventing dissolution of sulfur dioxide in water (2). A constant level of water in the gasholder is kept up by controlling water layer in the water tank (3). The gas is mixed with air, supplied by a compressor (4). The feed rate of sulfur dioxide and air is regulated by appropriate rheometers (5, 6). The gas mixture, produced in a mixer (7) is directed to the reactor (11), heated by a furnace (12). The temperature in the reactor is regulated by a potentiometer (13). The installation is equipped with samplers (8, 14).
When it is necessary to prepare the model gas mixture with vapors, a certain amount of water with the help of a syringe in a gas supply line (9) is supplied and is heated by a furnace (10) and supplied to the mixer (7). The gas mixture is supplied to the reactor from below. The reactor consists of a cylindrical glass (depending on operating temperature from molybdenum or quartz glass) vessel with a spherical perforated partition wall in the lower part and is equipped with a thermocouple well, it has bottom and top side tubes for supplying and discharging the gas mixture
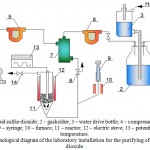 |
Figure 1: Schematic technological diagram of the laboratory installation for the purifying of gas mixture from sulfur dioxide |
Preparation of sorbent
The thermal treatment of thermophosphorus slag has been carried out in the electric stove with automatic temperature regulation. The period of preliminary calcination was considered as equal to 2 hours. The weight of thermophosphoric slag has been dried in thermostat at 120-150°C and then has been placed into the stove, and started heating. Upon reaching the predetermined temperature the heating time has been set. Prepared in such way sorbent has been stored in glass vessels with a glass stopper, i.e. there was not access to moisture and air.
Sorbent characterization
The results of mineralogical, X-ray and infrared spectroscopic analyses show that the thermophosphorous slag does not practically contain the individual oxides mixture. The initial sorbent, that has a light gray color, with a specific gravity of 2,86 g/cm3, hygroscopic moisture of 0,37%, a bulk density of 1,74 g/cm3 and grain size of 0,074-2,5 mm, is subjected to the pre-grinding for obtaining the homogeneous mass and more reactive surface. Chemical composition of termophosphorus slag is shown in Table 1 and an electronic image is presented in Figure 2.
Table 1: Chemical composition of the thermophosphorus slag
| No | Chemical formula | Content in % mass |
| 1 | SiO2 | 32.67 |
| 2 | Al2O3 | 4.90 |
| 3 | TiO2 | 0.25 |
| 4 | Fe2O3 | 3.90 |
| 5 | CaO | 41.92 |
| 6 | MgO | 3.85 |
| 7 | Р2О5 | 1.81 |
| 8 | SO3 | 0.90 |
| 9 | FeO+ MnO | 6.91 |
| 10 | K2O | 2.89 |
| Total: | 100 |
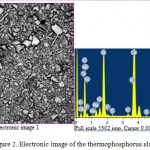 |
Figure 2: Electronic image of the thermophosphorus slag |
The studied sorbent in amount of 20 g and spherical nozzles are loaded into the reactor through the top side of tube. In the load condition the sorbent occupies the inter-packed space in the reactor. The gas mixture supplied under the slight excessive pressure, passing through the perforated partition wall in the reactor, tends to the upward by the way of the low effort closer to the outer wall of the reactor. At that, exerting pressure on the spherical nozzles from the side of walls, it is rolled up from the walls in radial direction. At that the sorbent occupies freed after the shift of the nozzle space. Multiple repetition of such displacement of the nozzle and sorbent simulates “a boiling bed”. Meanwhile, since level of the nozzle is considerably higher than level of the sorbent in the inter-packed space, entrainment of the adsorbent by the escaping gas mixture is negligible. Furthermore, the upper lateral aperture, by which the purified gas mixture enters, is filled with a glass fiber, which acts as a reflective trap.
20 g of the freshly calcined thermophosphorus slag by 0.25-0.50 mm fraction has been placed into the reactor. The gas mixture has been passed through the adsorbent layer at the constant volume velocity in 9000 h-1 and temperature in 100, 150, 200, 300, 400, 500, 600, 700, 800°C. The results are presented in Table 2.
Table 2: The influence of calcination temperature of the thermophosphorus slag on the degree of purification of the model gas mixture and dynamic capacity of the sorbent
| No | Temperature of the experiment, оС | Purification degree from sulfur dioxide, % vol. | Amount of sorbed dioxide from the beginning of adsorption,% mass | Reduction time of the purification degree till 0-10%, min. |
| a) calcination temperature is 500 ° C | ||||
| 1 | 100 | 32 | 2.73 | 25 |
| 2 | 150 | 38.5 | 4.42 | 40 |
| 3 | 200 | 50 | 6.82 | 55 |
| 4 | 300 | 52.3 | 6.91 | 45 |
| b) calcination temperature is 800°С* | ||||
| 5 | 200 | 35 | 2.90 | 50 |
| 6 | 300 | 35 | 3.02 | 30 |
| 7 | 400 | 46 | 8.45 | 65 |
| 8 | 500 | 55 | 8.36 | 55 |
| 9 | 600 | 35 | 1.72 | 20 |
| 10 | 700 | 35 | 1.72 | 20 |
| 11 | 800 | 35 | 1.65 | 15 |
| c) calcination temperature is 1000°С | ||||
| 12 | 200 | 60 | 7.74 | 60 |
| Duration of the calcination in all experiments is 2 hours | ||||
Results and discussion
The character of the sorption on the samples, differing by the mode of preliminary heat treatment is different. Thus, the maximum degree of adsorption on these adsorbents is observed at different temperatures. In the case of the sorbent, calcined at 500 °C, the maximum cleaning degree is observed at a temperature of 200 °C, and on a sample with calcination of 800 °C, 400-500 °C, respectively (Figure 4). They are also differed by the magnitude of dynamic capacity. Apparently, it is caused by the fact that various compounds, entering into the composition of phosphorus slag wastes participate in the process of sorption.
For the process temperature of 200 °C on the sorbent, calcined at 800 °C, sulphur dioxide concentration of 9,65%, a sulphur – capacitance is equal to 2,90 % wt. With the increase of the process temperature up to 500 °C, a sulphur – capacitance increases up to 3,36% wt. However, further increasing the temperature of the experiment up to 600 °C a sulphur – capacitance decreases up to 1,72% wt. It is probably occurs due to oversaturation of the sorbent as a result of oxidation of sulphide compounds.
Calcination temperature of the sorbent, which equal to 1000 °C allows to obtain a more active sorbent. It manifests 60% degree of cleaning even at 200 °C. However, high energy costs by calcination are not justified at an insignificant difference between the degrees of cleaning in the presence of sorbents, calcined at 500 °C and at 1000 °C. With the increase of calcination temperature from 500 °C up to 1000 °C, a sulphur- capacitance increases from 6,32 up to 7,74% wt.
Thus, a preliminary search of optimum conditions of heat-treatment of the sorbent allows to consider that the most suitable temperature of ore calcination is 500 °C (Fig.3), the cleaning process – 200°C or 800 ° C at the process temperature of cleaning of 500 °C.
The experimental results show that the highest activity of the sorbent calcined at 500°C is developed at the sorption temperature in 200°C (Fig. 4). At the further increase of temperature the sorption degree is not changed, and working time of the sorbent is reduced. This phenomenon may be the result of influence of adsorption-desorption factors on the process. From the practical point of view, low temperature is more convenient for development of technological scheme of the process. Consequently, temperature in 200 ° C can be considered as the most appropriate temperature of the process.
 |
Figure 3: Change of the purification degree and amount of the sorbed SO2 in time for different temperatures of the experiment |
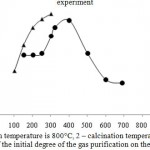 |
Figure 4: Dependence of the initial degree of the gas purification on the experiment temperature |
Bond strength of the adsorbed molecules with surface complex depends on the nature of its connection with substrate. It is the strongest one in the case of Fe2O3 – MgO compositions (He et al., 2008; He et al., 2004; Eid and Ammar, 2011), and in the case of Fe2O3 – SiO2 (Przepiórski et al., 2012) it is the weakest one.
Despite the fact that concentration of iron in the samples has not been changed, and specific surface was the greatest for silica gel, the sorption activity of Fe2O3 – SiO2 system was the lowest. From the works (Yao et al., 2008) it follows that mainly surface iron complexes with distorted octahedral symmetry of coordination sphere are formed in Fe2O3 – SiO2 compositions at low activation temperatures. It follows from this, that such complexes are not active in the direct chemisorption of sulfur dioxide. At the same time, MgFe2O3 phase and solid solution of Fe3+/MgO (He et al., 2008; He et al., 2004; Eid and Ammar, 2011; Yao et al., 2008), in which iron ions are difficult to reach, are mainly formed in Fe2O3 – MgO system. Nevertheless, activity of these samples was high. Fe2O3 – Al2O3 system, where iron complexes are on the surface, possessed greater activity (Yao et al., 2008; Komarov, 1977). As a result of the carried out researches we can make the following conclusion: a lot of surface and volume structures differing by symmetry of the coordination sphere of iron ions are formed in the course of iron supported systems thermal activation. The thermal phosphoric slag also contains minor amounts of the surface structures, but the main part of iron is in its matrix of complex formations. The adsorption of sulfur dioxide on Fe2O3 – supported systems and tailings is carried out on the multi-stepped mechanism. The part of SO2 is chemisorbed on the surface of iron complexes with formation of sulfite structures (Ya-nan et al., 2014; Ferrizz et al., 2002; Centi et al., 1992; Kirk-Othmer, 1991; Kaldybayev et al., 2013). We suggest that in the presence of moisture these surface complexes are oxidized by atmospheric oxygen till the corresponding sulfates. The main part of sulfur dioxide is chemisorbed on strongly basic centers of magnesium, calcium and aluminum oxides with formation of apparently similarly corresponding (surface) sulfates (He et al., 2004; Ya-nan et al., 2014; Ferrizz et al., 2002; Shakirov et al., 2013). The iron complexes at high temperatures actively participate in the activation of molecular oxygen, with the help of which catalytic oxidation of SO2 to SO3 and metal sulfites into the corresponding sulfates is carried out (He et al., 2004; Shakirov et al., 2013; Shakirov et al., 2012). Highly dispersed iron oxides of non-stoichiometric composition play the great role in the chemisorption and catalytic oxidation of SO2. Super-stoichiometric oxygen that was in the composition of these oxides is the active participant in the oxidation reaction of SO2.
According to the analysis results of the mineralogical composition produced by calcined at 500 °C and the saturated SO2, as shown by immersion researches aggregate carbonate-goethite-mica – limonite grains are dominated (≈85%). In these grains calcite (Np <1,519) are clearly fixed by the places.
The grains of quartz (Ng-Np» 0,01 и 1,570 > Ng > Np >1,531) are present in a small quantities (≈5%).
In the sorbent saturated by sulphate ions, sulphate grains with optical properties, characteristic for botriogen are also established by immersion analysis:
Ng = 1,574 ± 0,005
Nр = 1,525 ± 0,005 and
Ng – Np = 0,049 ± 0,01
These data for botriogen determined by us well fit with the literature data by the optical characterization of this mineral [000]:
Ng = 1,579 – 1,582,
Np = 1,522 – 1,523 and
Ng-Np – 0,055-0,059.
Number of clearly botriogen grains in immersion preparations makes about 10%. However, it is possible that the smallest botriogen inclusions are also present in the aggregate carbonate-limonite grains, which is optically veiled by limonite that does not reduce their chemical activity.
Limonite (goethite and hydrogoethite) is established by the brown color in the reflected light. Besides, nontransparent black grains are present in a small quantites, which likely are wustite (Shakirov et al., 2012) or iocite (FeO). Latter by literature data is unstable and is easily transformed into maghemite and magnetite (Shakirov et al., 2012). By the results of conducted researches, we can conclude that probably, the presence of minerals such as ankerite, dolomite and pyrite in thermo – phosphoric slags plays important role for the formation of activity of sorbent.
During the calcination, at the expense of these minerals oxides MgO, FeO and Fe2O3 are formed in thermo – phosphoric slags, which interacting with SO2 and vapors of H2O form sulphates. It must be emphasized that in our experiments, the formed SO2 during the decomposition of pyrite does not lose similarly vapor H2O and CO2 during the decomposition of carbonates, and is directly bind as sulphates in thermo – phosphoric slags. A such process character occupies place, apparently, when the sorbent is calcined at 500 °C for 2 hours. With increasing of calcining time up to 3-4 hours, sorbent loses its activity. We can assume that during the first two hours of calcining at the expense of pyrite and ankerite, iocite (FeO) is mainly formed, which during the longer calcining is transformed into maghemite (Fe2O3) and magnetite (Fe2O4) (Shakirov et al., 2012). The last two minerals do not practically interact with SO2 at a temperature of 200 °C, apparently, explains by the decrease of the sorption properties of thermo phosphoric slag, calcined for more than 2 hours.
As far as during cleaning of gas from sulphur dioxide, metal oxides of sorbent are converted into the corresponding sulphates, and the degree of cleaning is sharply reduced. Regeneration of sorbent is achieved by thermal catalytic decomposition of sulphates in the air. Regeneration temperature is gradually increased with increasing multiplicity of regeneration. Sorbent withstand a large number of thermal regeneration.
The decomposition reaction of the formed sulphates is one of the main processes, and at this the reactive oxides are formed on the surface of the sorbent. If a gas, containing sulphur dioxide is subjected to the cleaning, and concentrated gases can be obtained as a result of intensive decomposition of the formed metal sulphates, which are suitable for the production, for example sulphuric acid. At such statement of tasks, the sorbent can be used for many times, but the decomposition temperature of the metal sulfates is unequal.
The experiments by cleaning of gas mixture with the use of intermediate regenerations were carried out at a temperature of 200 °C using a sorbent, calcined at 500 °C. The initial degree of cleaning was 50%, a reducing time of the degree of cleaning is below 10% at 55 min., while sulphur capacitance of sorbent was 6.82 % wt. The spent sorbent was subjected to regeneration at a temperature of 500 °C for 2 hours. After such treatment of the spent sorbent, it proved to be unsuitable for cleaning of subsequent portions of the gas.
This phenomenon is apparently stipulated by the following. Basis of acting thermo phosphorus slag when cleaning are oxides of metals such as Al, Ca and Mg, which when cleaning above mentioned oxides, interacting with sulphur dioxide in the presence of oxygen and water vapor are converted into sulphates. Such sulphated thermo – phosphoric slag can be applied as a precious coagulant for wastewater treatment.
Conclusion
Influence of the preliminary activation process parameters on the quality of the gas emission purification from sulfur dioxide at the calcination temperatures (in the range from 100°C to 800°C), presence of water vapors in the gas mixture (0 and 10%), concentration of sulfur dioxide entering for the purification of gas mixture (in the range from 0.3 to 0.7% by vol.) has been studied. The chemisorption of sulfur dioxide with formation of sulfites, which pass into the sulfates in the air, occurs at the optimal parameters of the process. At the high temperatures, the chemisorption process is intensified at the expense of catalytic participation of metal oxides.
Thus, the possibility to purify waste (including flue) industrial gases from sulfur dioxide using the thermophosphorus slag as the sorbent has been studied. Regeneration of the spent sorbent and its futher use is the following perspectives of research work.
Acknowlegements
We would like to express our gratitude for support from the M.Auezov SKSU and Committee of science of the Ministry of Education and Science of the Republic of Kazakhstan (No. 275 of February 04, 2014).
References
- Ling, Z., Xinyong, L., Qidong, Z., Zhenping, Q., Deling, Y., Shaomin, L., Xijun, H. and Guohua, C. (2010). Synthesis, characterization and adsorptive performance of MgFe2O4 nanospheres for SO2 removal. (Vol. 184(1-3), pp. 704-709, DOI: 10.1016/j.jhazmat.2010.08.096). Journal of Hazardous Materials.
- Jin-an, W., Ze-lin, Z. and Cheng-lie, L. (1999). Pathway of the cycle between the oxidative adsorption of SO2 and the reductive decomposition of sulfate on the MgAl2-xFexO4 catalyst. (Vol. 139(1), pp. 31–41, DOI: 10.1016/S1381-1169(98)00186-1). Journal of Molecular Catalysis A: Chemical.
- Bennici, S., Gervasini, A., Ravasio, N. and Zaccheria, F. (2003). Optimization of tailoring of CuOx species of silica alumina supported catalysts for the selective catalytic reduction of NOx. (p.107 107 (22), pp 5168–5176 DOI: 10.1021/jp022064x). Journal of Physical Chemistry B.
- Smirnov, M.Y., Kalinkin, A.V., Pashis, A.V., Sorokin, A.M., Noskov, A.S., Kharas, K.C. and Bukhtiyarov, V.I. (2005). Interaction of Al2O3 and CeO2 surfaces with SO2 and SO2 + O2 studied by X-ray photoelectron spectroscopy. (Vol. 109, pp. 11712-11719, DOI: 10.1021/jp0508249). Journal of Physical Chemistry.
- Bretschneider, B. and Kurfurst, J. (1989). Air pollution control technology. Transl. from English. Leningrad, Chemistry Press.
- Li, J., Hao, J., Fu, L. and Zhu, T. (2004). High efficiency of noble metal and metal oxide catalyst systems for the selective reduction of NO with propene in lean exhaust gas. (pp. 30, DOI: 10.1023/B:TOCA.0000029732.81475.09). Topics in Catalysis.
- Chmielarz, L., Kus´trowski, P., Dziembaj, R., Cool, P. and Vansant, E.F. (2006). Catalytic performance of various mesoporous silicas modified with copper or iron oxides introduced by different ways in the selective reduction of NO by ammonia. (pp.62: 369, DOI: 10.1016/j.apcatb.2005.09.004). Applied Catalysis B: Environmental.
- He, C., Paulus, M., Chu, W., Find, J., Nickl, J.A. and Koehler, K. (2008). Selective catalytic reduction of NO by C3H8 over CoOx/Al2O3. An investigation of structure-activity relationships. (Vol. 131, p. 305, DOI: 10.1016/j.cattod.2007.10.024). Catalysis Today.
- He, H., Zhang, C. and Yu, Y. (2004). A comparative study of Ag/Al2O3 and Cu/Al2O3 catalysts for the selective catalytic reduction of NO by C(3)H(6). (Vol. 90, p. 191, DOI: 10.1016/j.cattod.2004.04.026). Catalysis Today.
- Eid, Kh.M. and Ammar, H.Y. (2011). Adsorption of SO2 on Li atoms deposited on MgO (1 0 0) surface: DFT calculations. (Vol. 257(14), pp. 6049–6058, DOI: 10.1016/j.apsusc.2011.01.122). Applied Surface Science.
- Przepiórski, J., Czyżewski, A., Kapica, J., Moszyński, D., Grzmil, B., Tryba, B., Mozia, S. and Morawski, A.W. (2012). Low temperature removal of SO2 traces from air by MgO-loaded porous carbons. (Vol. 191, pp. 147–153, DOI: 10.1016/j.cej.2012.02.087). Chemical Engineering.
- Yao, S., Pan, H., Zhang, Y. and Li, W. 2008. Promotion of MgO addition on SO2 tolerance of Ag/Al2O3 for selective catalytic reduction of NOx with methane at low temperature. (Vol. 9(5), pp. 796, DOI:10.1016/j.catcom.2007.09.002). Catalysis Communications.
- Komarov, V.M., 1977. Adsorbents and their properties. Minsk. Science and Technology Press.
- Ya-nan, S., Tao, L., You, T., Li, C. and Shan, H. (2014). Effect of sulfation on the performance of Fe2O3/Al2O3 catalyst in catalytic dehydrogenation of propane to propylene. (Vol. 244, pp. 145–151, DOI:10.1016/j.cej.2014.01.047). Chemical Engineering.
- Ferrizz, R.M., Gorte, R.J. and Vohs, J.M. (2002). TPD and XPS investigation of the interaction of SO2 with model ceria catalysts. (Vol. 82, pp. 123-129, DOI: 10.1023/A:1020512713021). Catalysis Letters.
- Centi, G., Passarini, N. and Perathoner, S. (1992). Combined DeSOx/DeNOx reactions on a copper on alumina sorbent-catalyst. 1. Mechanism of sulfur dioxide oxidation-adsorption. (Vol. 31, pp. 1947-1955, DOI: 10.1021/ie00008a016). Industrial and Engineering Chemistry Research
- Kirk, O. (1991). Encyclopedia of Chemical Technology. New York.
- Kaldybayev, A.B., Mirzayev, A.A., Zhantasov, K.T., Shakirov, B.S. and Baklanov, M.R. (2013). Using of phosphoric industry wastes as a sorbent for purification of sulfur-containing gases. (pp: 393-398). In the Proceedings of the International conference.
- Shakirov, B.S., Kaldybayev, A.B., Mirzayev, A.A., Kaldykozov, T.A. and Saipov, A. (2013). A method of sorption purification of waste gases from sulfur dioxide. Kazakhstan Patent 79339.
- Shakirov, B.S., Kaldybayev, A.B., Mirzayev, A.A. (2012). Development of technologies for adsorption-catalytic-chemisorption purification of gas emissions from sulfur dioxide. (No. 1492, pp. 77). Research report.

This work is licensed under a Creative Commons Attribution 4.0 International License.





