How to Cite | Publication History | PlumX Article Matrix
Ifah Munifah1, Titi Candra Sunarti2, Hari Eko Irianto3 and Anja Meryandini1
1Department of Biology, Faculty of Mathematics and Natural Sciences, Institut Pertanian Bogor, Dramaga Campus, Bogor 16680, Indonesia. 2Department of Agroindustrial Technology, Faculty of Agricultural Engineering and Technology, Bogor Agricultural University, Bogor 16680, Indonesia 3Research center for Fisheries Management and Conservation
DOI : http://dx.doi.org/10.13005/bbra/1862
ABSTRACT: Indonesia is known as the second seaweed producer in the world after China. Gracilaria sp seaweed is an important commodity in industry as raw material to produce agar and its derivate products. Solid wastes of seaweed processing industry (SWA) contain considerable amounts of cellulose. Cellulolytic bacteria were screened and isolated from the solid wastes of agar seaweed processing Industry in Malang, East Java, Indonesia. Among those isolates, LA4P strains showed higher potential for practical uses and identified as Bacillus pumilus strains by morphological, physiological, and biochemical characterization and 16S rRNA gene analysis. The optimum incubation time Bacillus pumilus LA4P in CMC broth (0.20 U/mL, 60 hours) relative shorter than 1% SWA broth (0.26 U/mL, 108 hours). The maximum enzyme production obtained using 2.5% SWA (0.34 U/mL). The test resulted that crude enzyme from Bacillus pumilus has a relatively high activity in the pH range 5-7 buffer at 0.23 U / mL and a temperature of 40 0C. The thermal stability assay indicated that the activity was stable at 30 to 600C after 15 minutes minutes incubation and it lost activity at 80 0C after 60 minutes incubation. The production patterns of cellulose degrading enzymes were investigated during cell culture. HPLC analysis confirm the degradation of these SWA substrates into soluble sugars. The isolated strains produced CMCase, Avicelase, β-glucosidase, and cellobiase enzymes, suggesting synergic cellulolytic systems in Bacillus pumilus LA4P.
KEYWORDS: solid waste; Seaweed; cellulolytic bacteria; Bacillus pumilus LA4P
Download this article as:| Copy the following to cite this article: Munifah I, Sunarti T. C, Irianto H. E, Meryandini A. Biodegradation of Solid Wastes of Agar Seaweed Processing Industry by Indigenous Cellulolytic Bacillus Pumilus LA4P. Biosci Biotech Res Asia 2015;12(3) |
Introduction
Seaweed is a source of abundant biomass and renewable, indicating its potential use as an alternative raw material for bioenergy production, and often referred to as the third generation biomass1,2,3. Seaweeds differ significantly from terrestrial plants, interm of chemical composition, physiology, and morphology. Carbohydrate content of macroalgae are relatively abundant and varied, that account of carbohydrates seaweed green, red, and brown, respectively by 25-50%, 30-60%, and 30-50% 4. Prospects of seaweed processing industry is still not matched by handling the processing of solid waste.
Red seaweed Gracilaria verrucosa, has been used for production of agar and bioethanol 3,5. The algae harvested at various time durations resulted in extraction of 27–33% agar. The leftover pulp was found to contain 62–68% holocellulose, which on enzymatic hydrolysis was done using commercial cellulase from Trichoderma reesei (ATCC 26921) and β-glucosidase from Aspergillus niger (Novozyme 188) were yielded 0.87 g sugars/g cellulose. The enzymatic hydrolysate on fermentation with Saccharomyces cerevisiae produced ethanol with a yield of 0.43 g/g sugars.
The mass balance evaluation of the complete process demonstrates that developing biorefinery approach for exploiting the red algae Gracilaria verrucosa, could be commercially viable. As terrestrial plants, seaweed contain high cellulose. The cellulose accounts for 30% to 50% of the total, while the seaweed’s component of cellulose ranges between 2-23% depending on its type 6.
Cellulose is generally found in the plant cell walls that are difficult to degraded in nature. It only a few of microorganisms are capable of degrading plant cell walls through cellulose hydrolysis. Bacteria and fungi can’t use this cellulose directly, therefore they secrete cellulase. Anaerobic bacteria, will secrete selulosom which is stick them on the surface of cellulose 7.
Cellulose is a carbohydrate that is abundant in nature. It is a linear polysaccharide composed of repeated glucose units bound at 1.4 acetal bond and forms a major component of cell walls both in higher plants as well as a low level. Cellulose is one of abundant renewable biopolymers in nature which has potential to be converted into value added product. It can be biodegraded by cellulase produced by cellulolytic microbe. In recent years, cellulosic biomass (cellulose and hemicellulose), resulted from processing of agricultural and forest waste, waste paper, and seaweed processing industry waste has been developed as a source of sugar for further fermentation into ethanol. Seaweed is a potential source of cellulosic material and is very useful as a raw material. Cellulose was first extracted from seaweed of the class Valonia, Halicystis, Cladophora and also some brown seaweed. Cellulose crystal in nature contained as a mixture of two crystalline cellulose α (as chain has a triclinic structure) and cellulose β (as two chain monoclinic structure). Cellulose derived from algal species consisting of porous cellulose hollow like a network that sets it apart from plant cellulose 8.
Several previous studies on hydrolysis of seaweed waste have been done using a strong acid, thermal treatment, a commercial enzyme, and the addition of cultured yeasts Trichoderma resei, Saccharomyces cerevisiae 3,9,10,11,12,13. Commercial enzyme and microbial cultures are relatively expensive and difficult to obtain. Therefore the whole cell or enzyme based hydrolysis will be economic if using the enzyme production from process utilizing local raw material as the substrat. The high cost of substrat is generally limiting factor in the production of the enzyme 14, therefore the selection of low cost substrate becomes very important. The application of cellulolytic bacteria to degrade seaweed solid waste is seen as a strategy to produce valuable product from solid wastes of agar seaweed processing industry. This study aims to utilize solid wastes of agar seaweed processing industry as source of indigenous cellulolytic and as a substrate for the production of cellulase enzymes.
Materials and Method
Sources of strain
Bacterial strain Bacillus pumilus LA4P, isolated from decaying industrial solid seaweed processing wastes (agar) Malang, East Java. It was cultured in a medium containing SWA or CMC as substrat (10 g/L); KH2PO4 (1.0 g/L); K2HPO4 (1.9g/L); MgSO4.7H2O (0.5 g/L); NaCl (0.5 g/L); FeSO4.7H2O (0.01 g/L); MnSO4.H2O (0.01 g/L); NH4NO3 (0.3 g/L); yeast extract (2g/L); and Agar (12.0 g/L).
Source of substrat SWA
Solid waste of agar seaweed processing industry was choosen as the carbon substrate in this study because their abundance in agar’s industry. It was collected from CV.Agar Sari Malang, East Java, Indonesia. Solid seaweed wastes were ground, sieved through a 2 mm screen for uniform particle size, and stored at 20C for further use. The final size of the samples was 200 mesh (75 µm).
Growth curve
Bacillus pumilus LA4P was grown in broth medium containing 1% SWA as major carbon source. Liquid culture were grown with vigorous aeration of 120 rpm at 37 0C. The bacterial growth was monitored by total plate count and the activity extracellularly produced cellulose was measured per 12 hours. LA4P was also grown in broth medium containing CMC and LIA broth medium, and the growth was monitore by spectrophotometric measurement at OD600nm.
Determination of optimum concentration SWA as major carbon source
To determine optimum concentration of SWA as carbon source, starter cultures were prepared by transferring Bacillus pumilus LA4P cells using an inoculation loop from the SWA agar plates to 100 mL of SWA liquid medium, supplemented with SWA at various concentration 0,5%; 1%, 1,5%; 2%; 2,5%; and 3%. The initial pH of medium was adjusted to 7.0 in 500 mL Erlenmeyer flasks. The flasks were further incubated on a shaking incubator/waterbath at 150 rpm for 8 days at 37 0C. Culture samples were taken every 24 h during incubation, and their cell free supernatants were obtained by centrifugation (10,000×g, 10 min) and analyzed for cellulolytic activities.
Production Cellulolytic Enzyme
Starter cultures were prepared by transferring cells using an inoculation loop from the SWA agar plates to 100mL of 2.5% SWA liquid medium. The initial pH of the medium was adjusted to 7.0 in 500mL Erlenmeyer flasks. After four days shaking incubation at 37 0C, aliquots of 2 mL starter cultures were seeded into 200 mL of SWA liquid medium in 500 mL flasks. The flasks were further incubated on a shaker at 150 rpm for 4 days at 37 0C. Supernatants were obtained by centrifugation (10,000×g, 10 min) and assayed for cellulolytic enzyme activities at various pH and certain temperature to determine stability.
Enzyme Activity Assay
CMCase activity was measured by incubating 0.2 mL of enzyme solution with 0.5mL of 1% (w/v) carboxymethyl cellulose, prepared in 50 mM citrate buffer (pH 4.5), and 0.3mL of 50 mM citrate buffer (pH 4.5) for 30 min at 37 0 C. The reducing sugars liberated were estimated by the 3,5-dinitrosalicylic acid (DNS) method. The enzyme reaction was stopped by the addition of 3 mL DNS reagent (dinitrosalicylic acid 1 g, NaOH, 16 g, potassium sodium tartarate 300 g, and distilled water up to 1 L) to the above 1 mL reaction mixture, boiled in capped glass tubes for 5 min, and cooled in cold water, and then optical density was measured at 540 nm. The CMCase activity was determined using a calibration curve for d-glucose. One unit of CMCase activity was defined as the amount of enzyme that released 1 μmol of reducing sugars as glucose equivalents min−1.
pH optimum of crude enzyme
To estimate the optimal pH of the crude enzyme, activity was measured using 1% (w/v) CMC as the substrate at 37 0C in 100 mM citrate buffer (pH 3 and 4), citrate-phosphat (pH 4, 5, and 6), buffer phosphate (pH 6, 7, and 8). The crude enzyme stability at different pH level was determined by measuring the activity after incubating the enzymes for 30 minutes at 37 0C at pH 3.0 to pH 8. After preincubation, the enzyme solution was subjected to the normal cellulase assay using DNS method’s.
Optimum Temperature of crude enzyme
The optimal temperature of crude enzyme Bacillus pumilus LA4P was determined. 500 µL of appropriate concentration of enzyme were added to 500 µL of 1% CMC in 50 mM citrate buffer, were added and incubated at various temperatures (30, 37, 40, 50, 60, 70, 80 0C) for 30 min. The activity was then measured to DNS method.
Stability temperature of crude enzyme
Enzyme stability of crude enzyme from Bacillus pumilus LA4P was studied over the temperature range of 30 to 80 0C. Stability temperature of crude enzyme was determined using standard CMC assay. The crude enzyme from Bacillus pumilus LA4P was incubated for 15 minutes and 60 minutes at different temperatures and then added to 500 µL of 1% CMC (in buffer citrate phosphate pH 6.0). The enzyme activity was then measured according to DNS method.
Substrate Specifity
Hydrolytic ability from Bacillus pumilus LA4P against 0.1 % (w/v) agar, solid waste of agar industry (SWA), Avicel, cellobiosa, xylan, filter paper (FP), CMC, and p-nitrophenol β-D-glucopyranoside (pNPG) in 50 mM citrate phosphate buffer (pH 6) were determined to evaluate substrate specifity of crude enzyme.
Avicelase activity was measured by incubating 0.5 mL of enzyme solution with 0.1% (w/v) of Avicel, a microcrystalline cellulose, as substrate and 1.5 mL of 0.1M sodium acetate buffer (pH 5.0) for 1 h at 500C. After incubation, the reaction mixture was centrifuged at 10,000×g for 5 min, and then 1 mL of the supernatant was taken to determine reducing sugars by the DNS method. One unit of Avicelase activity was defined as the amount of enzyme that released 1 μmol of reducing sugars as glucose equivalents min−1.
Filter paper-hydrolytic (FPase) activity was measured by a procedure in which Whatman no. 1 filter paper was used as a substrate. Fifty milligrams of the substrate suspended in 1.5 mL of 50 mM citrate phosphate buffer (pH 6.0) was incubated with 0.5mL of enzyme solution at 50 0C for 2 h. After incubation, the reaction mixture was centrifuged at 10,000×g for 5 min, and then 1 mL of the supernatant was used for the determination of reducing sugars by the DNS method. One unit of FPase activity was defined as the amount of enzyme that released 1 μmol of reducing sugars as glucose equivalents min−1. β-glucosidase (or cellobiase) activity was measured by using ρ-nitrophenyl-β-D-glucopyranoside (pNPG) as a substrate. The hydrolysis of pNPG releases ρ-nitrophenol, a pigmented substance that can be measured spectrophotometrically at 400 nm. The reaction mixture, containing 0.5mL of 1mg/mL pNPG, 0.5mL of 50 mM citrate phosphate buffer (pH 6.0), and 0.5mL of enzyme solution, was incubated at 50◦C for 1 h. The enzyme reaction was stopped by adding 2mL of 1M Na2CO3, and the absorbance was measured at 400 nm. One unit of β-glucosidase activity was defined as the amount of enzyme that released 1 μmol of para-nitrophenol min−1.
Analysis of product hydrolysis using thin layer chromatography
Bacillus pumilus LA4P was grown in broth medium containing 2.5% (w/v) solid wastes of agar seaweed processing Industry (SWA) as major carbon source. Liquid culture were grown with vigorous aeration 120 rpm at 37 0C. Supernatant as the product of SWA hydrolysis were collected every 24 hours. Samples were centrifuge to remove the insoluble SWA. This followed by an analyzed using HPLC and thin layer chromatography. The supernatant were performed on Silica Gel F254 HPTLC plates (Merck) to detect carbohydrates. The plate were developed with 2:1:1 n-butanol:acetic acid:water solution, air dried and then heat at 110 0C. Degradation products were visualized by UV lamp at 254 nm.
Analysis of product hydrolysis using HPLC
The supernatant (crude enzyme) from Bacillus pumilus LA4P were vacuum filtered to remove contaminants using 0.2 µm fiter (Millipore) into glass vials and analyzed for monomeric and oligomeric sugars. The sugar analysis was performed in an Agilent 1200 High Pressure Liquid Chromatography (HPLC), fitted with Agilent Hi-Plex H, 7.7 × 300 mm, 8 µm. The column temperature was maintained at 80 0C and RID was maintained 550C during analysis. The mobile phase used was 0.005 M H2SO4 in water HPLC filtered. Gradient Isocratic, flow rate 0.7 mL/min, injection volume was 20 µL. The standard solutions and sugar consist of glucose, xylose, cellobiose, cellotriose, and cellotetraose.
Result and Discussion
One of the most prerequirement step for industrial production of large quantity of enzyme is potential microbial strains. Halo zone formation on agar medium is a qualitative indicator for the ability of cellulolytic microorganism to produce cellulose enzyme extracellularly15. Although initial research on cellulase was mainly focused on fungi, recently there has been increasing interest in cellulase production using bacteria because the bacterial growth is relatively faster compared with fungi. Figure 1 shows that isolates Bacillus pumilus LA4P cellulase enzyme activity is relatively more potent than other isolates in plate agar. The diameter of the halo zone is very useful for predicting the enzyme yield, as an aid to select strain with high level of polysaccharide (such as cellulose) degrading activities15.
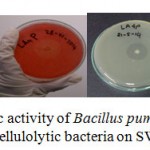 |
Figure 1: Screening for cellulolytic activity of Bacillus pumilus LA4P, photo image of clearing zone around colony of cellulolytic bacteria on SWA agar after 5 days |
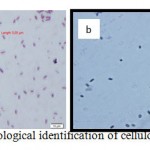 |
Figure 2: Morphological identification of cellulolytic bacterium |
Morphological examination showed the colour white, round irregular colony shape, surface colonies smooth, lobate colony edge, and shiny smooth colony appearance, indicating that the species is aerobic. Microscopically, this strain was found to be a gram positive motile bacillus (Fig. 2a). Malachite green staining showed oval spore at the central endospore of bacteria (Fig. 2b).
Table 1: Biochemical characteristics of LA4P strains from solid waste of agar seaweed processing industry
| Characteristic | Result | Substrat Utilization | Result |
| Amylase | + | D-Glucose | + |
| protease | + | L-arabinose | + |
| lipase | + | D-xylose | + |
| phosphatase | – | D-mannitolGalactose | + |
| Dnase | – | Fructose | + |
| Gelatinase | + | Mannose | + |
| Chitinase | + | Nitrate | + |
| Growth temperature | room temperature | Adonitol | – |
| oxidase | + | Dulcitol | – |
| catalase | + | Sorbitol | – |
| indole production | – | Inositol | – |
| Voges proskauer | + | Urea | – |
| citrate utilization | – |
Growth curve in media CMC and SWA
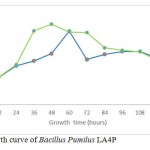 |
Figure 3a: Growth curve of Bacillus Pumilus LA4P |
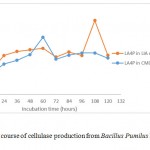 |
Figure 3b: Time course of cellulase production from Bacillus Pumilus LA4P in different media |
The growth curve in Fig. 3a and 3b indicated the presence of cellulose activity in the supernatant obtained from CMC and SWA broth as seen in Table 2. The optimum incubation time Bacillus pumilus LA4P in CMC broth (60 hours) relative shorter than 1% SWA broth (108 hours) (Fig. 3). Carboxylmethylcellulose is soluble, while SWA is insoluble complex substrate. The structurally complex of SWA makes incubation time longer than in comparison with soluble substrate.
Table 2: Cellulase activity of Bacillus pumilus LA4P in SWA and CMC broth
|
isolat |
Activity | Optimum incubation time
|
||
| CMC (U/mL) | SWA (U/mL) | CMC (jam) | SWA (jam) | |
| LA4P | 0.200317 | 0.25843755 | 60 | 108 |
Optimization percentage SWA substrate in growth media isolates LA4P
 |
Figure 4: Effect of different concentration source carbon on enzyme production by Bacillus pumilus |
The concentration effect (0.5-3%) of solid waste of agar seaweed processing industry (SWA) as carbon source towards the enzyme production was studied. The result showed that the concentration of SWA played a critical role in enzyme yield. The media was inoculated Bacillus pumilus LA4P with different concentration of SWA and incubated at 37 0C for 7 days. The optimum enzyme production was observed in SWA concentration range of 1.5% to 2.5% of SWA (Fig. 4). The maximum production obtained using 2.5% SWA (0.34 U/mL).
Table 3. Effect of concentration solid waste of agar seaweed processing industry (SWA) as the carbon source
| % SWA substrat | Incubation time (day) | Activity ( U/mL) |
| 0.5 | 5 | 0.20 |
| 1 | 5 | 0.20 |
| 1.5 | 2 | 0.198 |
| 2 | 3 | 0.17 |
| 2.5 | 4 | 0.34 |
| 3 | 6 | 0.20 |
The crude enzyme showed the highest activity on cellobiose and CMC. There was almost no hydrolysis activity observed on the substrate xylan, filter paper and p-NPG. We assumed that the crude cellulose from Bacillus pumilus LA4P consist of an endo-1,4 β-glucanase and cellobiohidrolase. The enzyme endo 1,4 β-glucanase and β-glucosidase work synergistically to degrade cellulose to glucose. Exo 1.4 β–glucanase enzyme cut off the ends of cellulose chains crystalline into reducing sugars prior to the activity of endo 1,4 β-glucanase enzyme that cleavages the amorphous site.
Table 4: Substrat specifity of cellulolytic crude enzyme from Bacillus pumilus LA4P
| 0.1% Substrates | Activity (U/mL) |
| Agar | 0.0880 |
| SWA | 0.1274 |
| Avicel | 0.1186 |
| cellobiosa | 0.1429 |
| Xylan | 0.0074 |
| FP | 0.00265 |
| CMC | 0.1344 |
| pNpG | 0 |
A reducing sugar would bind to DNS solution (dinitro salicylic acid) which produces an orange color. The greater the amount of reducing sugars produced, increasingly concentrated color reactant. The absence of activity paperase filters because very little possibility of enzyme activity 1.4 exo β -glukanase produced by Bacillus pumilus LA4P isolates. Each enzyme cellulase has specificity of the structure around the substrate. Differences in the specificity of the enzyme endoglucanase and cellobiohidrolase is not absolute because both enzyme can hydrolyze β-1,4 glucoside bond of amorphous cellulose.
The predominant activity in crude enzyme from Bacillus pumilus LA4P was cellobiohidrolase, but the crude enzyme also displayed carboxymethylcellulase (CMCase), avicelase, and hydrolysis well SWA substrat.
pH and Temperature optimum of crude enzyme
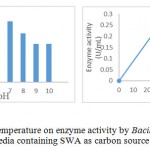 |
Figure 5: Effect pH and temperature on enzyme activity by Bacillus Pumilus LA4P in enzyme production media containing SWA as carbon source |
Temperature and pH were important parameters that influenced enzyme activites. Temperature and pH of the enzyme depends on the type and source of enzyme. Determination of optimum pH and temperature was intended to obtain the proper pH and temperature, in which the enzyme works with the highest activity. The test resulted that crude enzyme from Bacillus pumilus LA4P has a relatively high activity in the pH 6 buffer at 0.23 U / mL and a temperature of 40 0C (Fig. 5). Activity of enzyme tend to increases in proportion to the increase in incubation temperature and reaches a maximum value at 40 0C. Optimum pH studies another researcher of Bacillus pumilus cellulose was active on pH 6 at temperature 500C 15,17 and pH 6.5 at similar temperature 16. Information of temperature and pH optimum the activity of this enzyme is very useful for the application of this enzyme due to the material to be hydrolyzed.
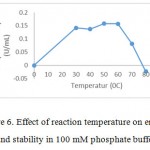 |
Figure 6: Effect of reaction temperature on enzyme activity aand stability in 100 mM phosphate buffer at pH7.0 |
Thermal stability constitutes a very important property when intend to apply the industrial importance. Thermostability study of Bacillus pumilus LA4P crude enzyme was assessed by incubating the crude enzyme at the temperature range of 30-800C for 15 minutes and 60 minutes of incubation. The crude enzyme from Bacillus pumilus LA4P was incubated in 50 mM citrate phosphate buffer, pH 6.0, for 15 minutes and 60 minutes at different temperatures and then added to 500 µL of 1% CMC in 50 mM citrate phosphate buffer (pH 6.0). The thermal stability assay indicated that the activity was stable at 30 to 600C after 15 minutes minutes incubation and it lost activity at 800C after 60 minutes incubation (Fig. 6). Thermal stabilitiy studies of another Bacillus pumilus S124A reported that the carboxymethl cellulose was stable about 84.4% at 50 0C17.
Analysis of product hydrolysis
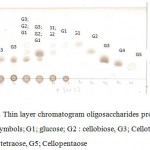 |
Figure 7: Thin layer chromatogram oligosaccharides produced by LA4P. Symbols; G1; glucose; G2 : cellobiose, G3; Cellotriose, G4; cellotetraose, G5; Cellopentaose |
From the TLC result in Figure 8, it was observed that the crude supernatant was able to degrade the insoluble substrate SWA to form smaller products cellotriose in the second day of incubation time. Initially, after 48 hours, a very small amount of cellotriose (G3) were formed during incubation. The product cellotriose increased with incubation time until 5th day. After the 4th and 5th days of incubation, the TLC and chromatogram HPLC results showed the presence of the hydrolysis product indicated as G3, G2 and small amount of G1.
Table 5: Retention time TLC and HPLC of hydrolysis product
| P 2.5% | standar | Rf TLC | Retention time HPLC | ||||||
| Rf TLC standar | Rf1 | Rf2 | Rf3 | tR standar | tR1 | tR2 | tR3 | ||
| G1 | 0.55-0.64 | 9.761 | |||||||
| G2 | 0.41-0.52 | 8.296 | |||||||
| G3 | 0.27-0.41 | 7.69 | |||||||
| G4 | 0.21-0.33 | 7.429 | |||||||
| G5 | 0.16-0.20 | 7.317 | |||||||
| Day 1 | |||||||||
| Day 2 | 0.27-0.40 (G3) | 7.769 | |||||||
| Day 3 | 0.27-0.40 (G3) | 7.924 | |||||||
| Day 4 | 0.27-0.40 (G3) | 0.41-0.49 (G2) | 0.53-0.62 (G1) | 7.975 | 8.37 | 9.675 | |||
| Day 5 | 0.30-0.36 (G3) | 0.37-0.50 (G2) | 0.54-0.63 (G1) | 7.975 | 8.37 | 9.675 | |||
| Day 6 | 0.41-0.48 (G2) | 0.55-0.62 (G1) | 8.229 | 9.68 | |||||
| Day 7 | 0.55-0.62 (G1) | 9.683 | |||||||
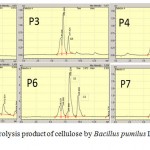 |
Figure 8: HPLC of hydrolysis product of cellulose by Bacillus pumilus LA4P incubation time for 2 – 7 day |
Conclusion
Since pure commercialized cellulose is expensive as substrate, solid waste of agar seaweed processing industry (SWA) can be used as a good alternative for cellulose production. The ability of microorganisms to convert cellulose into simple carbohydrates have been known for decades. Here we reported our results on the hydrolysis of solid waste of agar seaweed processing industry (SWA) by indigenous cellulolytic Bacillus pumilus LA4P. The test resulted that crude enzyme from Bacillus pumilus LA47 has high activity using 2.5% SWA (0.34 U/mL). The optimum pH and temperature was 6, 400C. It lost activity at 800C after 60 minutes incubation. HPLC analysis confirm the degradation of these SWA substrates into soluble sugars Suggesting synergic cellulolytic systems in Bacillus pumilus LA4P, this strains produced CMCase, Avicelase, β-glucosidase, and cellobiase enzymes.
Acknowledgment
This work was supported by Research Centre for Marine and Fisheries Product Processing and Biotechnology, Jakarta Indonesia. We also acknowledge to Dr. Ekowati Chasanah and Ir.Pujoyuwono M, MSc.
References
- Singh A, Nigam PS, Murphy JD. 2011. Mechanism and Challenges in Commercialisation of Algal biofuels. Biores Technol. 102:26-34.
- Jang J, Cho Y, Jeong G, Kim S. 2012. Optimization of Sacharification and ethanol production by simultaneous saccharification and fermentation (SSF) from seaweed Saccharina japonica. Bioprocess Biosyst Eng. 35:11-18
- Kim HT, Yun EJ, Wang D, Chung JH, Choi IG, Kim KH. 2013. High temperature and low acid pretreatment and agarase treatment of agarose for the production of sugar and ethanol from red seaweed biomass. Biores 136:582–587
- Jung KA, Lim SR, Kim Y, Park JM. 2013. Potentials of macroalgae as feedstocks for biorefinery. Biores 135:182–190
- Kumar, S., Gupta, R., Kumar, G., Sahoo, D., & Kuhad, R. C. 2013. Bioethanol production from Gracilaria verrucosa, a red alga, in a biorefinery approach. Bioresource Technology, 135, 150–156. doi:10.1016/j.biortech.2012.10.120
- Kim GS, Myung KS, Kim YJ, Oh KK, Kim JS, Ryu HJ, dan Kim KH. 2008. Methode of producing biofuel using sea algae. Seoul: World Intelectual Property Organization
- Wilson DB. 2011. Microbial diversity of cellulose hydrolisis. Current Opinion in Microbiology. 14:1-5.
- Siddhanta AK, Prasad K, Meena R, Presad G, Mehta GK, Chhatabar MU. 2009. Profiling of cellulose content in Indian seaweed species. Biores Technol. 100:6669–6673.
- Kim YS, Chae SW, Park DH, Sunwoo C. 2010. Pretreatment of Gelidium amansii for the production of bioethanol. Korean Chem. Soc. l31:511-517
- Kim NJ, Li H, Jung K, Chang HN, Lee PC. 2011. Ethanol production from marine algal hydrolysates using Escherichia coli KO11. Biores Technol. 102:7466–7470.
- John RP, Anisha GS,Nampoothiri KM, Pandey A. 2011. Micro and macroalgal biomass: A renewable source for bioethanol. Biores Technol. 102:186–193.
- Yanagisawa M, Nakamura K, Ariga O, Nakasaki K. 2011. Production of high concentrations of bioethanol from seaweeds that contain easily hydrolyzable polysaccharides. Process Biochem. 46: 2111–2116
- Ruiz HA, Jasso RM, Fernandes BD, Vicente AA, Teixeira JA. 2013. Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: A review. Renewable and Sustainable Energy Reviews. 21:35–51.
- Ariffin H, Hassan M, Shah UK, Abdullah N, Ghazali FM, and Shirai Y. 2008. Production of bacterial endoglucanase from pretreated oil palm empty fruit bunch by Bacillus pumilus EB3. J. Biosci Bioeng 106:231-236.
- Saini, Jitendra Kumar, Tewari, L. 2012. Simultaneous Isolation and Screening of Cellulolytic Bacteria : Selection of Efficient Medium. Journal of Pure and Applied Microbiology, 6, 1339–1344.
- André, O. S. L., Maria, C.Q., Fungaro, M.H.P., Fernando, D.A., Walter, M.J., Welington L.A., Márcio, C.S., Aline, A.P. and João, L.A. 2005 Molecular characterization of a β-1,4-endoglucanase from an endophytic Bacillus pumilus strain. Applied Microbiology and Biotechnology, 68: 57-65.
- Natesan Balasubramanian and Nelson Simões. 2014. Bacillus pumilus S124A carboxymethyl cellulase; a thermo stableenzyme with a wide substrate spectrum utility. International Journal of Biological Macromolecules 67 132–139. http://dx.doi.org/10.1016/j.ijbiomac.2014.03.014

This work is licensed under a Creative Commons Attribution 4.0 International License.





