How to Cite | Publication History | PlumX Article Matrix
Yulia Evgenyevna Kravchenko1, Stepan Petrovich Chumakov1, Elena Ivanovna Frolova1,*, Yuriy Nikolaevich Lezhnin1,2, Dmitry Sergeevich Kravchenko1,2
1Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences, 117997, Russian Federation, Moscow, GSP-7, Ulitsa Miklukho-Maklaya, 16/10
2Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, 119991 Moscow, Russia
DOI : http://dx.doi.org/10.13005/bbra/1884
ABSTRACT: Development of novel highly selective anticancer therapeutics usualy requires large amount of target protein that serves as a bait for selection. In many cases these proteins posess posttranslational modifications or features, that could not be replicated in procaryotic expression systems, thus requiring more laborous and expensive expression in mammalian cells. Transfection-based eucaryotic producents, on the other hand, are unstable and gradually lose production yields. We developed novel lentivirus-based expression system for fast and simple production and purification of recombinant proteins in mammalian cells. Our expression system allows easy selection of producent cells with flow sorting, antibody-free assessment of secreted protein concentration in cellular media and fast two-stage purification of target protein devoid of any tag sequences. We implemented our expression system for fast production of highly efficient mammalian producent of CD44 receptor extracellular part and subsequent purification of properly folded and glycosylated protein
KEYWORDS: Lentiviral vectors; CD44; tagRFP; protein expression
Download this article as:| Copy the following to cite this article: Kravchenko Y. E, Chumakov S. P, Frolova E. I, Lezhnin Y. N, Kravchenko D. S. Lentivirus-Based System for Fast Expression and Purification of Recombinant Proteins and its Implementation for Production of Human CD44 Extracellular Part. Biosci Biotechnol Res Asia 2015;12(3) |
| Copy the following to cite this URL: Kravchenko Y. E, Chumakov S. P, Frolova E. I, Lezhnin Y. N, Kravchenko D. S. Lentivirus-Based System for Fast Expression and Purification of Recombinant Proteins and its Implementation for Production of Human CD44 Extracellular Part. Biosci Biotechnol Res Asia 2015;12(3). Available from: https://www.biotech-asia.org/?p=4097> |
Introduction
Production of pure recombinant proteins in appropriate quantity is an essential requirement for many research projects. Nowadays there is wide selection of expression systems available for large-scale recombinant protein production. The most popular approach is to express the protein of interest in E.coli or yeast strains. Microbial systems, especially E. coli, have several advantages that are associated with low cost of establishing a production strain, quick production cycle, easy in-process control, and higher productivity compared to mammalian expression systems [1]. However, several disadvantages exist that limit usage of prokaryote systems; proper microbial expression is quite challenging for large complex proteins that contain multiple subunits, disulfide bonds or different post-translational modifications such as protein glycosylation [2]. Efficient expression of these difficult protein targets requires mammalian cells as hosts. Expression systems utilizing mammalian cells for manufacturing of recombinant proteins are able to introduce proper protein folding and post-translational modifications which are important for complete biological activity.
Today more than 50% of all recombinant proteins for pharmaceutical trials and science applications are produced in mammalian cells [3]. Most of these proteins are expressed in immortalized chinese hamster ovary (CHO) or Human embryonic kidney (HEK 293) cells. Generally, recombinant genes are delivered into cells by transfection with plasmid DNA [4, 5]. This method is known as transient expression. Selection of stable and efficiently expressing clones after transient transfection is crucial for successful protein production. On the downside, transient expression systems are expensive, often inefficient, and prone to loss of expression over time. In contrast with that, delivery of recombinant genes by lentiviral particles is more laborous during initial introduction of expression cassette and less complicated on successive steps due to irreversible and virtually unlimited integration in genome, that allows faster selection of recombinant clones and eliminates the threat of expression decline over time [6].
We constructed recombinant lentiviral vector containing sequence of extracellular part of CD44 receptor. CD44 protein is a cell surface glycoprotein expressed in a large number of mammalian cell types. CD44 is a multifunctional molecule involved in cell differentiation, cell proliferation, angiogenesis and other cellular functions. All these biological properties of CD44 are essential for physiological activities of normal cells, but are also frequently associated with pathological activities of cancer cells [7]. Higher levels of expression of CD44 receptor are observed in various types of cancer cells including breast, lung, prostate, ovarian, cervical, and colorectal cancers [8-12]. Various cellular events including cancer development are typically accompanied by changes in cellular glycosylation profiles. Glycan changes in malignant cells may occur in different forms. These changes might be linked with loss of expression or excessive expression of certain structures, or with persistence of incomplete or truncated structures. Therefore CD44 represents a frequent target for development of novel therapeutic agents [13]. These tasks require production of sufficient quantity of properly folded and glycosylated CD44 as a bait. Here we describe recombinant lentivirus vector suitable for efficient production and purification of CD44 protein in mammalian expression system.
Materials and Methods
Table 1: List of the used primers
| Target | Primer sequence (5’-3’) | Restriction sites | |
| EF1alpha promoter | F | GAGAGAATCGATAGCGTGAGGCTCCGGTGCCCGTCAGTGG
|
ClaI |
| EF1alpha promoter | R | AGAGAGTCTAGAAGGAATTCTCACGACACCTGAAATGGAAGAAAAAAAC | XbaI |
| CD44 receptor | F | AGAGAGTCTAGAACCATGGACAAGTTTTGGTGGCACGCAGCCTG | XbaI |
| CD44 receptor | R | AGAGAGGGATCCGGAGGGATTCTGTCTGTGCTGTCGGTGATC | BamHI |
| TEV-tagRFP | F | AGAGAGAGGATCCGGAGAATCTTTATTTTCAGGGCAGCATGGTGTCTAAGGGCGAAG | BamHI |
| tagRFP | R | CTCTCTGTCGACTAATGATGATGATGATGATGATGATGATGATGGCTACCGGAGCCGCTGCCATTAAGTTTGTGCCCCAG | SalI |
Cell culture
Аdherently growing human embryonic kidney (HEK) cell lines HEK 293T and HEK 293 were cultured in Dulbecco’s modified Eagle’s medium (DMEM, high glucose (4.5 g/L) with 2 mM L-glutamine) supplemented with 10% (v/v) heat inactivated fetal calf serum (FCS) (Thermo scientific, USA) and 1% (v/v) of a 10.000 U/mL penicillin and 10 mg/mL streptomycin stock solution (Paneco, Russia) at 37°C, 5% CO2.
RNA isolation and cDNA preparation
Total RNA was extracted from MCF7 cells using TRIzol isolation reagent (Invitrogen, USA). The RNA was reverse transcribed using oligo (dT)20 by incubation with 200 U of SuperScript III reverse transcriptase at 50 oC at 50 min in the presence of 25 mM MgCl2, 10x RT Buffer, 10 mM dithiothreitol (DTT), 40 U of RNase OUT (RNase inhibitor) and 10 mM dNTP Mix. Obtained cDNA was used for amplification of EF1alpha promoter region and CD44 gene.
Construction and identification of recombinant pLEF1a-CD44-TEV-tagRFP Vector
The coding sequence of EF1alpha promoter was amplified from cDNA of HEK 293T cells by Tersus HS DNA Polymerase (Evrogen, Russia) and ClaI and XbaI restriction sites were added to 5’ and 3’ end of PCR products (primer sequences in Table 1). The digested (ClaI + XbaI) PCR products and pL-NP-MCS plasmid were then ligated to introduce new promoter region in lentiviral vector named pL-EF1alpha-NP-MCS.
The coding sequence of CD44 receptor was amplified from cDNA of MCF7 cells and inserted into pL-EF1alpha-NP-MCS vector between XbaI and BamHI restriction sites (primer sequences in Table1).
The TagRFP sequence was amplified from pTagRFP-N (Evrogen, Russia) by two step PCR reaction with forward primer containing TEV protease digestion site. Amplified sequence was cloned into the vector between BamHI and SalI restriction sites.
Lentiviral packaging and virus collection
Twenty-four hours prior to transfection, the HEK 293T cells growing at 70% confluency were trypsinized, and the cell density was adjusted to 1.0×106 cells/mL with complete culture medium. The cells were reseeded into 10-cm cell culture dishes and cultured for 24 h prior to transfection. The cells were 75%–85% confluent on the day of transfection. The recombinant viral vector encoding the CD44 receptor and the three packaging plasmids pRSV-REV, pCMV-VSV-G and pCMV-GAG were co-transfected into HEK 293T cells using TurboFect reagent (Fermentas, USA) according to the manufacturer’s instructions. After 8 h of transfection, the cell culture medium was replaced with fresh DMEM medium supplemented with 2% FBS. After 24 h of transfection, the tagRFP expression was determined. After 48 h of transfection, the culture medium was collected and filtered through a 0.45-μM membrane to remove any cellular debris. After that PEG 8000 was added and supernatant was incubated on ice for 24 h. To obtain high virus titer, supernatant was centrifuged at 4000×g at 4 °C for 30 min and white precipitate was resuspended in 1 ml PBS.
Virus transduction and fluorescent cell selection
HEK 293 cells were seeded at 5×105 cells per well in 12 well plates in DMEM containing 10% FBS. After 24 h incubation, the cells were transduced with whole lentivirus stock. HEK 293 cells were then incubated 24-48 h after transduction prior to selection the RFP positive cells by cell sorting (BD FACS Vantage SE, USA).
Detection of Expression and Secretion of CD44 Protein in HEK 293 Cells
Detection of transgene mRNA expression by PCR analysis
Total RNA from HEK 293 cells infected with the pL-EF1alpha-CD44-TEV-RFP expression vector was prepared using TRIzol reagent (Invitrogen, USA) after viral transduction. cDNA was prepared as described previously. The transgene insertion was detected by PCR amplification with EF1alpha promoter F and tagRFP R primers. The resulted fragments were separated by 1.0% agarose gel electrophoresis.
SDS-PAGE electrophoresis and Western blotting
Collected fractions were mixed with sample buffer and run on a 10% SDS–PAGE gel under reducing conditions. The separated proteins were transferred to PVDF (Polyvinylidene fluoride) membrane (Hybond GE Healthcare, USA). PVDF was blocked with PBS containing 3% BSA and incubated with primary monoclonal mouse antibodies specific to CD44 extracellular domain for 1 h at room temperature followed by a three steps of washing by PBS containing 0.1 % Tween 20 and incubated with a peroxidase-labelled rabbit anti-mouse antibodies. Peroxidase activity was visualized using tetramethylbenzidine substrate (TMB) (Aurora, USA).
Results
Design and construction of lentivirus expression vector
For determination of coding sequences of EF1alpha promoter and CD44 extracellular part we used GenBank database. Recombinant vector and primers (Table 1) were virtually predicted and constructed by SnapGene software. After PCR amplification, EF1 alpha, CD44 and tagRFP PCR fragments were successively digested and cloned to pL-NP-MCS vector, which was digested by the same sites (Figure 1).
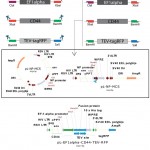 |
Figure 1: Schematic representation of construction of pL-EF1alpha-CD44-TEV-RFP expression cassette |
Recombinant lentivirus vector was verified by PCR and restriction analysis (Figure 2). Also, plasmid was sequenced with using of EF1alpha promoter and tagRFP primers. Sequencing was carried out by Evrogen biotechnology (Evrogen, Russia).
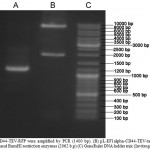 |
Figure 2: (A) CD44-TEV-RFP were amplified by PCR (1400 bp). (B) pL-EF1alpha-CD44-TEV-tagRFP vector was digested by ClaI and BamHI restriction enzymes (2062 b.p) (C) GeneRuler DNA ladder mix (Invitrogen, USA) |
Packaging of the pL-EF1alpha-CD44-TEV-RFP lentivirus and titer assay:
HEK 293T cells were co-transfected with the transfer plasmid pL-EF1alpha-CD44-TEV-RFP and the packaging helper plasmids pCMV-GAG, pCMV-VSV-G and pRSV-REV. RFP was detected after 16 h after transfection (Figure 3). The viral titer was calculated as proportion of fluorescent (RFP) positive cells that was determined by flow cytometry. Virus titer was 3.5×109 U/mL.
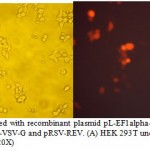 |
Figure 3: HEK 293T cells transfected with recombinant plasmid pL-EF1alpha-CD44-TEV-RFP and the packaging helper plasmids pCMV-GAG, pCMV-VSV-G and pRSV-REV. (A) HEK 293T under light microscope (20X); (B) HEK 293T under fluorescent microscope (20X) |
Virus transduction and fluorescent cell analysis:
CD44-tagRFP fusion was expressed 36 h after the HEK 293 cells were infected with lentiviral particles, and cells expressing transgene were observed under a fluorescence microscope (Figure 3). This confirms that the EF1alpha-CD44-TEV-RFP expression cassette was successfully transduced into HEK 293 cells. Then tagRFP positive cells were selected by fluorescence-activated cell sorting (FACS) (Figure 4).
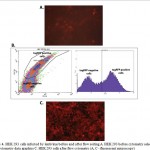 |
Figure 4: HEK 293 cells infected by lentivirus before and after flow sorting A. HEK 293 before cytometry selection B. Flow cytometry data graphics C. HEK 293 cells after flow cytometry (A, C – fluorescent microscopy) |
Detection of transgene integration into cellular genome and expression of CD44-TEV-RFP mRNA in HEK 293 cells:
To confirm insertion of lentiviral transgene into the genome of tagRFP-positive cells, PCR on gDNA was performed using primers cPPT and WPRE (Table 1), which were designed to anneal to the vector region outside of the promoter and protein coding sequence. Gel electrophoresis of PCR products showed the expected 2776 bp band (Figure 2) corresponding to the lentiviral expression cassette, whereas no bands were detected in the uninfected cells.
To establish expression of stable CD44-TEV-RFP mRNA production in transgenic cells, we isolated total RNA and prepared cDNA. After that cDNA was amplified by PCR. The 1452 bp fragment (Figure 2) corresponding to the CD44-TEV-tagRFP sequence amplified by PCR analysis was present only in cDNA obtained from lentivirus-transduced HEK 293 cells but not from uninfected HEK 293 cells.
Protein production analysis
Following infection of HEK 293 host cells and their selection, passaging in serum-free medium Hybris (Paneco, Russia) took two weeks before culture supernatant could be tested for recombinant CD44-TEV-RFP secretion. Cultivation was carried out at constant shaking to avoid cells aggregation. After 10 days of cultivation, cells were harvested, counted and transfered at 500 mL fresh medium (Hybris, without FBS), at concentration 1×105-2×105 cells/mL. After 4 days cultivation, cells were spun down and supernatant was filtered through 0.45 membrane and loaded on a Ni-NTA superflow column (5PRIME, Germany).
Metal chelate affinity chromatography was performed using BioRad BioLogic Pathfinder system (BioRad, USA). After affinity chromatography the eluted soluble CD44-TEV-tagRFP fusion protein was tested using SDS-PAGE gel electrophoresis and Western blot analysis. A typical elution profile of the protein fractions collected from a Ni-sepharose column is shown in Figure 5. The specific 80 kDa band eluted in fraction D was purified to homogenity (Figure 5, lane D of elution). Western blot analysis confirmed that elution fraction (D lane, Figure 5) contained CD44-TEV-RFP recombinant protein. The total volume of collected fractions (C and D) was 20 mL.
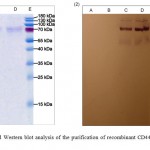 |
Figure 5: SDS-PAGE and Western blot analysis of the purification of recombinant CD44-TEV-RFP-His10 on a Ni-NTA Sepharose |
Discussion
Еstablishing mammalian cell lines that stably express recombinant proteins with high efficiency remains one of the major priorities for biopharmaceutical manufacturing and scientific research. In this study, we successfully developed a novel lentivirus-based vector for effective expression of various recombinant proteins in mammalian cells. Lentiviral vector was chosen as the most advanced transgene delivery system. Features of lentivirus-based delivery system allow to achive stable integration of expressing cassette in the cellular genome and long-term expression of the transgene. To show the efficiency of our system we expressed extracellular domain of human CD44 cell receptor. CD44 belongs to group of highly glycosylated proteins, which makes it appropriate as a model protein for expression in mammalian cells.
In order to optimize the lentiviral vector for production of recombinant proteins in the HEK 293 cell line we evaluated 4 various promoter regions (data not shown) with respect to expression strength. We have compared expression levels of the tagRFP protein under control of CMV (cytomegalovirus promoter) , NP (p53 promoter region), SV40 (Simian vacuolating virus 40 promoter region) and EF1alpha (Human elongation factor-1 alpha promoter) promoters. It was found that the highest level of tagRFP expression is observed when using EF1alpha promoter region. Thereby, the EF1alpha promoter was chosen for further development of our lentiviral vector. Also plasmid backbone includes other core elements like a Woodchuck Hepatitis Virus Posttranscriptional Regulatory Element (WPRE), central polypurine tract (cPPT), polyadenylation signal (polyA) and Rev response element (RRE). Incorporation of such regulating sequences into lentivirus vector provides increased transduction efficiency and transgene expression in the cell.
For the assessment of efficiency of transduction and further selection of transduced cells we used tagRFP fluorescent protein. Usage of fluorescent proteins as a selectable markers has significant advantages over the luciferase and drug resistant proteins . The main advantage of this approach is ability to efficiently select transgenic cells by fluorescence-activated cell sorting . Furthermore we fused CD44 and tagRFP protein, thus the level of brightness of selected cells directly correlated with CD44 expression level. In order to separate fusion proteins and isolate pure CD44 fraction, TEV protease digestion site was incorporated between CD44 and tagRFP. Utilizing this vector system we established protein-expressing HEK 293 cell line in less than one week.
In summary, these findings provide valuable data that will help optimize production of different recombinant proteins in HEK 293 or other cell lines. Presented lentiviral protein expressing system is simple and highly efficient and may be adapted for bio-therapy protein manufacturing and various other biotechnology applications.
Conclusions
We developed novel system for lentivirus-mediated expression and purification of recombinant proteins in mammalian cells. This system is characterized by stable protein production, easier determination of protein yield in culture media by measurement of fluorescence levels and simple purification procedure with positive metal-chelate affiniti chromatography, TEV protease digestion and subsequent fluorescent tag removal with negative metal-chelate affiniti chromatography. We further tested our expression system by establishing effective producent of recombinant human CD44 receptor extracellular part and purifying expressed protein from cellular media.
Acknowledgements
The work funded by MESR contract №14.607.21.0062 (RFMEFI60714X0062).
References
- Rosano, G. L. and Ceccarelli, E. A. (2014) Recombinant protein expression in Escherichia coli: advances and challenges, Frontiers in microbiology. 5, 172.
- Dalton, A. C. and Barton, W. A. (2014) Over-expression of secreted proteins from mammalian cell lines, Protein science : a publication of the Protein Society. 23, 517-25.
- Wurm, F. M. (2004) Production of recombinant protein therapeutics in cultivated mammalian cells, Nature biotechnology. 22, 1393-8.
- Geisse, S. and Fux, C. (2009) Recombinant protein production by transient gene transfer into Mammalian cells, Methods in enzymology. 463, 223-38.
- Baldi, L., Hacker, D. L., Adam, M. and Wurm, F. M. (2007) Recombinant protein production by large-scale transient gene expression in mammalian cells: state of the art and future perspectives, Biotechnology letters. 29, 677-84.
- Nayerossadat, N., Maedeh, T. and Ali, P. A. (2012) Viral and nonviral delivery systems for gene delivery, Advanced biomedical research. 1, 27.
- Naor, D., Nedvetzki, S., Golan, I., Melnik, L. and Faitelson, Y. (2002) CD44 in cancer, Critical reviews in clinical laboratory sciences. 39, 527-79.
- Olsson, E., Honeth, G., Bendahl, P. O., Saal, L. H., Gruvberger-Saal, S., Ringner, M., Vallon-Christersson, J., Jonsson, G., Holm, K., Lovgren, K., Ferno, M., Grabau, D., Borg, A. and Hegardt, C. (2011) CD44 isoforms are heterogeneously expressed in breast cancer and correlate with tumor subtypes and cancer stem cell markers, BMC cancer. 11, 418.
- Lokeshwar, B. L., Lokeshwar, V. B. and Block, N. L. (1995) Expression of CD44 in prostate cancer cells: association with cell proliferation and invasive potential, Anticancer research. 15, 1191-8.
- Sillanpaa, S., Anttila, M. A., Voutilainen, K., Tammi, R. H., Tammi, M. I., Saarikoski, S. V. and Kosma, V. M. (2003) CD44 expression indicates favorable prognosis in epithelial ovarian cancer, Clinical cancer research : an official journal of the American Association for Cancer Research. 9, 5318-24.
- Wang, S. J. and Bourguignon, L. Y. (2011) Role of hyaluronan-mediated CD44 signaling in head and neck squamous cell carcinoma progression and chemoresistance, The American journal of pathology. 178, 956-63.
- Belov, L., Zhou, J. and Christopherson, R. I. (2010) Cell surface markers in colorectal cancer prognosis, International journal of molecular sciences. 12, 78-113.
- Shah, V., Taratula, O., Garbuzenko, O. B., Taratula, O. R., Rodriguez-Rodriguez, L. and Minko, T. (2013) Targeted nanomedicine for suppression of CD44 and simultaneous cell death induction in ovarian cancer: an optimal delivery of siRNA and anticancer drug, Clinical cancer research : an official journal of the American Association for Cancer Research. 19, 6193-204.

This work is licensed under a Creative Commons Attribution 4.0 International License.





